��Ŀ����
��һ��ʯ��ʯ��Ʒ�ijɷ���CaCO3��SiO2������С��ͬѧ��100g�����5�μ��뵽35gʯ��ʯ��Ʒ�У���֪SiO2�������ᷴӦ�����õ��粿�����ݣ�
������5��ʵ������Һ���10%��CaCl2��Һ�����������Һ�м���������ʯ��ʯ��ĩ����ȫ��Ӧ����ˣ�����㣺��ʱ����Ҫ����Һ�м���ˮ���ٿˣ�������ʵ���������Һ��ʧ���Բ��ƣ�
| ���� | ��1�� | ��2�� | ��3�� | ��4�� | ��5�� |
| �������������/g | 20 | 20 | 20 | 20 | 20 |
| ʣ����������/g | 30 | 25 | 20 | 15 | 15 |
���������ݱ������ݷ���ǰ4��ÿ�μ���20gϡ�����������������5g��˵��ÿ20g����ϡ������5g̼���ǡ����ȫ��Ӧ��������ټ�20gͬ����ϡ��������������ټ���˵��ʣ���15g����ΪSiO2��35gʯ��ʯ��Ʒ��̼��Ƶ�����Ϊ20g���μӷ�Ӧ��ϡ��������Ϊ80gʣ��20g��Ȼ�����20g̼��ƺ�80gϡ���ᷴӦ���ɵ��Ȼ��Ƶ�����������ɵ��Ȼ��Ƶ��������������20gϡ���ᷴӦ�����Ȼ��Ƶ���������20gϡ���ᷴӦ���ĵ�̼��Ƶ�����Ϊ5g��Ȼ������������������ļ��㹫ʽ���㼴�ɣ�
����⣺��35gʯ��ʯ��Ʒȫ���μӷ�Ӧ���ɵ��Ȼ��Ƶ�����Ϊx�����ɶ�����̼������Ϊa��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 111 44
20g x a
=
x=22.2g
=
a=8.8g
��20gϡ����ȫ��Ӧ�����Ȼ��Ƶ�����Ϊy�����ɶ�����̼������Ϊb��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 111 44
5g y b
=
y=5.55g
=
b=2.2g
��100gϡ���ᷴӦ���ĵ�̼��Ƶ�����Ϊ25g�����Ը��������غ㶨�ɿ�֪���ɵ��Ȼ�����Һ������Ϊ125g-8.8g-2.2g=114g
������Һ���Ȼ��Ƶ�����Ϊ22.2g+5.55g=27.75g
������5��ʵ������Һ���10%��CaCl2��Һ����Һ������Ϊ
=277.5g����Ҫ����Һ�м���ˮ������Ϊ277.5g-114g=163.5g
CaCO3+2HCl=CaCl2+H2O+CO2��
100 111 44
20g x a
| 100 |
| 20g |
| 111 |
| x |
x=22.2g
| 100 |
| 20g |
| 44 |
| a |
a=8.8g
��20gϡ����ȫ��Ӧ�����Ȼ��Ƶ�����Ϊy�����ɶ�����̼������Ϊb��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 111 44
5g y b
| 100 |
| 5g |
| 111 |
| y |
y=5.55g
| 100 |
| 5g |
| 44 |
| b |
b=2.2g
��100gϡ���ᷴӦ���ĵ�̼��Ƶ�����Ϊ25g�����Ը��������غ㶨�ɿ�֪���ɵ��Ȼ�����Һ������Ϊ125g-8.8g-2.2g=114g
������Һ���Ȼ��Ƶ�����Ϊ22.2g+5.55g=27.75g
������5��ʵ������Һ���10%��CaCl2��Һ����Һ������Ϊ
| 27.75g |
| 10% |
������������Ҫ����ѧ�����û�ѧ����ʽ���м��������������������ʽ�ļ������������㲽�跱��ֻҪ�������⼴�ɼ���������

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
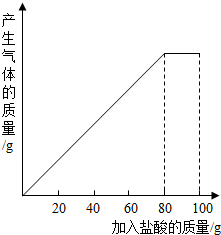 ��һ��ʯ��ʯ��Ʒ����Ҫ�ɷ���CaCO3������С��ͬѧ��100gϡ�����5�μ��뵽35gʯ��ʯ��Ʒ�У���֪���ʲ������ᷴӦҲ������ˮ�����õ����²������ݺ�ͼ������ϸ�۲����б����ͼ����㣺
��һ��ʯ��ʯ��Ʒ����Ҫ�ɷ���CaCO3������С��ͬѧ��100gϡ�����5�μ��뵽35gʯ��ʯ��Ʒ�У���֪���ʲ������ᷴӦҲ������ˮ�����õ����²������ݺ�ͼ������ϸ�۲����б����ͼ����㣺| ���� | ��1�� | ��2�� | ��3�� |
| �������������/g | 20 | 20 | 20 |
| ʣ����������/g | 30 | a | 20 |
��2��ʯ��ʯ��Ʒ��CaCO3��������Ϊ���٣��������ȷ��0.1%��
��3��10%��CaCl2��Һ����Ϊ·�汣ʪ����������5��ʵ������Һ���10%��CaCl2��Һ�����������Һ�м���������ʯ���ҷ�ĩ����ȫ��Ӧ����ˣ���ʱ����Ҫ����Һ�м���ˮ���ٿˣ�������ʵ���������Һ��ʧ���Բ��ƣ�
 ��һ��ʯ��ʯ��Ʒ�ijɷ���CaCO3��SiO2������С��ͬѧ��100g�����5�μ��뵽35gʯ��ʯ��Ʒ�У���֪SiO2�������ᷴӦ�����õ����²������ݺ�ͼ��
��һ��ʯ��ʯ��Ʒ�ijɷ���CaCO3��SiO2������С��ͬѧ��100g�����5�μ��뵽35gʯ��ʯ��Ʒ�У���֪SiO2�������ᷴӦ�����õ����²������ݺ�ͼ��| ���� | ��1�� | ��2�� | ��3�� |
| �������������/g | 20 | 20 | 20 |
| ʣ����������/g | 30 | a | 20 |
��1����2�μ��������aΪ
��2��ʯ��ʯ��Ʒ�и�Ԫ�ء�̼Ԫ�غ���Ԫ�ص�������Ϊ���٣����������������ȱ�ʾ��
��3��10%��CaCl2��Һ����·�汣ʪ����������5��ʵ������Һ���10%��CaCl2��Һ�����������Һ�м���������ʯ��ʯ��ĩ����ȫ��Ӧ����ˣ���ʱ����Ҫ����Һ�м���ˮ���ٿˣ�������ʵ���������Һ��ʧ���Բ��ƣ�
��һ��ʯ��ʯ��Ʒ�ijɷ���CaCO3��SiO2������С��ͬѧ��100g�����5�μ��뵽35gʯ��ʯ��Ʒ�У���֪SiO2�������ᷴӦ�����õ����²������ݣ�
������5��ʵ������Һ���10%��CaCl2��Һ�����������Һ�м���������ʯ��ʯ��ĩ����ȫ��Ӧ����ˣ�����㣺��ʱ����Ҫ����Һ�м���ˮ���ٿˣ�������ʵ���������Һ��ʧ���Բ��ƣ�
| ���� | ��1�� | ��2�� | ��3�� | ��4�� | ��5�� |
| �������������/g | 20 | 20 | 20 | 20 | 20 |
| ʣ����������/g | 30 | 25 | 20 | 15 | 15 |
��һ��ʯ��ʯ��Ʒ�ijɷ���CaCO3��SiO2������С��ͬѧ��100g�����5�μ��뵽35gʯ��ʯ��Ʒ�У���֪SiO2������ˮҲ�������ᷴӦ�����õ����±��������ݣ�
����㣺
��1����2�μ��������aΪ g��
��2��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ���٣�
��3��10%��CaCl2��Һ����·�汣ʪ����������5��ʵ������Һ���10%��CaCl2��Һ�����������Һ�м���������ʯ��ʯ��ĩ����ȫ��Ӧ����ˣ���ʱ����Ҫ����Һ�м���ˮ���ٿˣ�������ʵ���������Һ��ʧ���Բ��ƣ�
��4������35gʯ��ʯ��Ʒ�м��������������ʣ�����������仯��ϵ��ʾ��ͼ���ڡ��������ͼ����ͼ��
| ���� | ��1�� | ��2�� | ��3�� | ��4�� | ��5�� |
| �������������/g | 20 | 20 | 20 | 20 | 20 |
| ʣ����������/g | 30 | a | 20 | 15 | 15 |
��1����2�μ��������aΪ
��2��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ���٣�
��3��10%��CaCl2��Һ����·�汣ʪ����������5��ʵ������Һ���10%��CaCl2��Һ�����������Һ�м���������ʯ��ʯ��ĩ����ȫ��Ӧ����ˣ���ʱ����Ҫ����Һ�м���ˮ���ٿˣ�������ʵ���������Һ��ʧ���Բ��ƣ�
��4������35gʯ��ʯ��Ʒ�м��������������ʣ�����������仯��ϵ��ʾ��ͼ���ڡ��������ͼ����ͼ��
