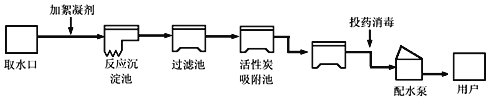
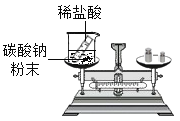
��Ŀ����
����Ŀ��A��B��C��D��E��F��G��H�������ʣ�����ͼ��ʾ��ϵ������A��һ�ְ���ɫ���壬B��G���Ǻ�ɫ���壬D��ʹ�����ǵ�ľ����ȼ��E��F������ɫҺ�壬H��һ����ʹ����ʯ��ˮ����ǵ����壬�ڢڵķ�Ӧǰ��B�������ͻ�ѧ���ʶ����ı䡣�������������Ϣ���ƶϳ���������
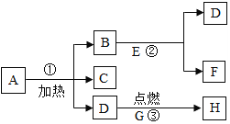
��1��д�����ʵĵĻ�ѧʽ��A_________�� B_________�� C________��
��2��B�ڢ��е�������______________________��
��3��д����Ӧ�ٵĻ�ѧ����ʽ��________________________
��4����֪G���ʾ��л�ԭ�ԣ����о�G���ʻ�ԭ����������ķ���ʽ��________
���𰸡�KMnO4 MnO2 K2MnO4 ������ 2KMnO4![]() K2MnO4+MnO2+O2�� C+2CuO
K2MnO4+MnO2+O2�� C+2CuO![]() 2Cu+CO2��
2Cu+CO2��
��������
A��һ�ְ���ɫ���壬����������ʹ�����ǵ�ľ����ȼ������D������D��������A�Ǹ�����أ�G������ȼ��������ʹ�����ʯ��ˮ����ǵ�����H������H�Ƕ�����̼��G��̼��B����ɫҺ�����ܲ�����������B�ڷ�Ӧǰ�������ͻ�ѧ���ʶ����ı䣬����B�Ƕ������̣�E�ǹ������⣬���ɵ�F��ˮ��������طֽ���������ء��������̺�����������C������أ�������֤���Ƶ���ȷ��
�ɷ�����֪��1��A��KMnO4��B��MnO2��C��K2MnO4��
��2��B�ڢ��е������Ǵ����ã�
��3����Ӧ���Ǹ�������ڼ��ȵ���������������ء��������̺���������ѧ����ʽΪ��2KMnO4![]() K2MnO4+MnO2+O2����
K2MnO4+MnO2+O2����
��4����֪G���ʾ��л�ԭ�ԣ�G���ʻ�ԭ����������Ļ�ѧ����ʽΪ��C+2CuO![]() 2Cu+CO2����
2Cu+CO2����

 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д� �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д�����Ŀ��ijʵ��С����3������غͶ������̻�������ʵ�飬���ȸû����t1ʱ���(���ʲ���Ӧ)����ȴ������ʣ�������������ظ����ϲ��������μ���t2��t3��t4ʱ�����ʣ��������������¼�������±���
����ʱ�� | t1 | t2 | t3 | t4 |
ʣ���������(g) | 2.68 | 2.36 | 2.04 | 2.04 |
(1)����t3ʱ���������Ƿ�ֽ���ȫ��_________(����������������)
(2)��ȫ��Ӧ���������������Ϊ_____________�ˣ�
(3)�û����������ص�����Ϊ_____��
����Ŀ��Ϊ̽��������˫��ˮ��H2O2���ֽ�Ĵ�Ч����ij�о�С����������ʵ�飺
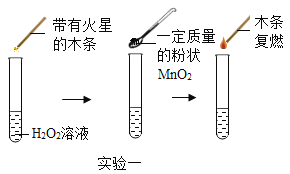
ʵ��һ��ͼ�е�ʵ���ܷ�֤��MnO2��˫��ˮ�ֽⷴӦ�Ĵ�������˵������_______��
ʵ�����ѧϰ�˴���֪ʶ��ij��ѧ̽��С�鿪ʼ����Ѱ���µĴ������о���ʵ �飺
��������⣩�������ܷ�������������Һ�ֽ�Ĵ���������ܣ����Ч����Σ�
��ʵ��̽����
ʵ�鲽�� | ʵ������ |
I���ֱ���ȡ5mL5%����������Һ����A��B��֧�Թ��У���A�Թ��м���ag��������ĩ�����ֱ���A��B��֧�Թ��в��������ľ�����۲����� | A�Թ��в������ݣ�������ľ����ȼ��B�Թ������������� |
II����A�Թ���û��������ʱ�����¼������������Һ�����Ѵ����ǵ�ľ�������Թܣ���˷���������飬�۲����� | ______________ |
III����ʵ��II�е�ʣ����С�Ĺ��ˣ�����������������ϴ�ӡ�������������� | ���ù�������______g |
IV�ֱ���ȡ5mL5%����������Һ����C��D��֧�Թ��У���C�Թ��м���ag��������ĩ����D�Թ��м���ag�������̷�ĩ���۲����� | D�Թ��в������ݵ����ʸ��� |
��ʵ����ۣ���1��ʵ��________�ڣ���ʵ�鲽��Ĵ��ţ���ʵ��II�Ĵ���Ϊ��II����֤������������������������ֽ�Ĵ�����
��ʵ�����ۣ�
��2��ʵ�����IV��Ŀ����______��
��3������ʵ��IV����������Եõ��Ľ����ǣ�__________��