��Ŀ����
5��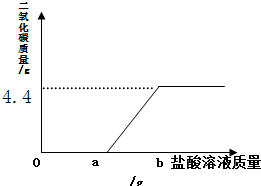 һƿ���õ��������ƹ����Ѿ������˱��ʣ�ij�о���ѧϰС��Ϊ��̽�����ʳ̶ȣ��������²��룺���ܲ��ֱ��ʣ�������NaOH�� Na2CO3�Ļ�������ȫ�����ʣ�������Na2CO3
һƿ���õ��������ƹ����Ѿ������˱��ʣ�ij�о���ѧϰС��Ϊ��̽�����ʳ̶ȣ��������²��룺���ܲ��ֱ��ʣ�������NaOH�� Na2CO3�Ļ�������ȫ�����ʣ�������Na2CO3��1�����ȶԹ���ijɷֽ���ȷ����ȡ�����������Թ��У���ˮ����ܽ⣬�ȼ���������BaCl2 ��Һ��������ɫ���������ú�ȡ�ϲ���Һ���ټ���CuSO4 ��Һ��������ɫ��״����������ʵ������ȷ���ù�����NaOH��Na2CO3�Ļ���
��2����ȡ14.6g �ù�����Ʒ����ƿ�У�������������Ϊ7.3%��ϡ���ᣬ����a��ʱ��ʼ�������壬����b��ʱ���岻�ٲ������õ�������ͼ������㣺
�ٸ���Ʒ��Na2CO3������
�ڸ���Ʒ��NaOH�ı��ʳ̶ȣ��ðٷֱȱ�ʾ��
����Ӧ����Һ������������
���� ��1������������BaCl2��Һ��������ɫ�������ð�ɫ������BaCO3��˵���ù���ɷ��к���CO32-���ټ���CuSO4��Һ��������ɫ��״����������ɫ��״������Cu��OH��2��˵���ù���ɷ��к���OH-��
��2������ͼʾ��֪���ɵĶ�����̼���������������ɵĶ�����̼��������Ϸ�Ӧ�Ļ�ѧ����ʽ���������̼���Ƶ���������������������Ƶ��������ɵ������Ȼ��Ƶ���������Ӧ����Һ������������
��� �⣺��1����������֪���ù�����Һ�к���CO32-��OH-��������ƿ���õ��������ƹ�����NaOH��Na2CO3�Ļ���
���NaOH��Na2CO3�Ļ���
��2���ٸ���ͼʾ��֪���ɵĶ�����̼������Ϊ4.4g��
����Ʒ��̼���Ƶ�����Ϊx�������Ȼ��Ƶ�����Ϊz����Ҫ���������Ϊw��
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 73 117 44
x w z 4.4g
$\frac{106}{x}=\frac{44}{4.4g}$
��ã�x=10.6g
$\frac{117}{z}=\frac{44}{4.4g}$
z=11.7g
$\frac{73}{w}=\frac{44}{4.4g}$
w=7.3g
������ʵ��������Ƶ�����Ϊy��
2NaOH+CO2�TNa2CO3+H2O
80 106
y 10.6g
$\frac{80}{y}$=$\frac{106}{10.6g}$
y=8g
δ���ʵ��������Ƶ�����Ϊ��14.6g-10.6g=4g��
����Ʒ��NaOH�ı��ʳ̶�Ϊ$\frac{8g}{8g+4g}$��100%=66.7%
����4g�������������ᷴӦ���ɵ��Ȼ��Ƶ�����Ϊp����Ҫ�Ȼ�������Ϊn
NaOH+HCl=NaCl+H2O
40 36.5 58.5
4g n p
$\frac{40}{4g}=\frac{36.5}{n}=\frac{58.5}{p}$
n=3.65g��p=5.85g
�����������������Ϊ$\frac{7.3g+3.65g}{7.3%}$=150g
��Ӧ����Һ����������Ϊ$\frac{11.7g+5.85g}{150g+14.6g-4.4g}$��100%=11.0%
�ʴ�Ϊ����10.6g
��66.7%
��11.0%��
���� Ҫ�����������Ŀ�����ȣ�Ҫ�������Ǹ��ݻ�ѧ��Ӧ����ʽ�ļ��㲽���ʽ���Լ���֮��ص�֪ʶ�ȣ�Ȼ���������������龰�������ѧ�����֪ʶ�ͼ��ܣ�ϸ�µط������Ⲣϸ�ĵ�̽��������������ĿҪ����������ѡ����ɣ�

 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�| A�� | 15g | B�� | 10g | C�� | 5g | D�� | 30g |
| A�� | ��ͭ | B�� | ���� | C�� | �� | D�� | ����ʯ |