��Ŀ����
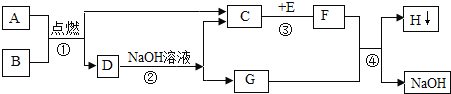
����Ŀ��������ͼ��ʾʵ��װ�ûش��й����⣺
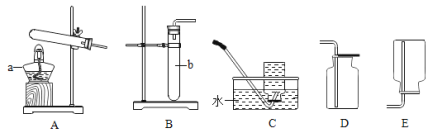
��1��д��װ���б�����������ƣ�a___________��
��2�� ������������ȼ�ϣ������ܶȱȿ���С��������ˮ��ʵ���ҳ���п����ϡ���ᷴӦ���Ƶã��仯ѧ����ʽΪ___________��
��3��ʵ���ҳ����Ȼ�粒������ʯ�ҹ��干������ȡ������NH3����������NH3��һ����ɫ���д̼�����ζ�����壬�ܶȱȿ���С��NH3��������ˮ����ˮ��Һ�ʼ��ԡ�
����ȡ������Ӧ�ķ���ʽΪ2NH4Cl+Ca(OH)2![]() CaCl2+2NH3
CaCl2+2NH3![]() +2X��X�Ļ�ѧʽΪ��______��
+2X��X�Ļ�ѧʽΪ��______��
����ȡ���ռ�NH3 ��Ӧ�ô���ͼ��ѡ��ķ���װ����___���ռ�װ����____�������ţ�
��NH3��һ�ּ������壬����ʱ����ѡ�����и�����е�____������ţ���
A �������ƹ��� B Ũ���� C ��ʯ��
���𰸡��ƾ��� Zn+H2SO4�TZnSO4+H2�� H2O A E B
��������
��1��д��װ���б�����������ƣ�a�ƾ��ơ�
��2�� ������������ȼ�ϣ������ܶȱȿ���С��������ˮ��ʵ���ҳ���п����ϡ���ᷴӦ���Ƶã��仯ѧ����ʽΪZn+H2SO4�TZnSO4+H2����
��3���پݻ�ѧ��Ӧǰ��ԭ�ӵ����ࡢ��Ŀ���䣬��Ӧǰ�е�ԭ��2������ԭ��8������ԭ��2������ԭ��1������ԭ��2������Ӧ���и�ԭ��1������ԭ��2������ԭ��6������X�Ļ�ѧʽΪH2O
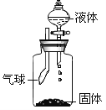
�����Ȼ�粒������ʯ�ҹ��干������ȡ������NH3�������ڹ�������ͣ���ѡ����װ��A�������ܶȱȿ�����������ˮ�������������ſ������ռ���
��Ũ�����백���ɷ�Ӧ���ʰ���������Ũ������