��Ŀ����
����Ŀ�������еĸ�Ԫ����Ҫ�����ڹ����������У��������ǻ�����ƾ���[Ca10 (PO4) 6 (OH) 2]��ʽ���ڣ�����Է�������Ϊ1004��ţ�̺����������գ���ţ���иƺ��ı������ʣ��ǽ��ǵ�����ʳƷ����ͼ��ij��ҵ��˾��ţ�̰�װ��ǩ�ϵIJ������֣����Ķ���ش���������:

��1����װ��ǩ��֬��>3.3g����ָ100 mLţ���к�֬������������Ϊ3.3g����ôһ��ţ���к�������______g (������0.01g) ��
��2���ǻ�������и�Ԫ�ص���������Ϊ_____ (������һλС��)��
��3��������ÿ��������Ҫ0.6g�ƣ�����Щ����90%����ţ�̣���һ����ÿ�����ٺ�___��ţ�̡�
���𰸡���1��0.28����2��39.8%����3��2
��������
��1��һ��ţ�̺���Ԫ�ص�������![]() ��
��
��2���ǻ�������и�Ԫ�ص�����������![]() ��
��
��3��0.6g��90%��0.28g��2�У�һ����ÿ������Ҫ��2��ţ�̡�

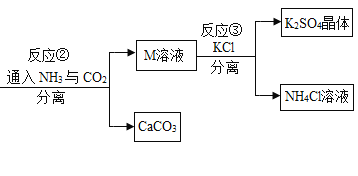
����Ŀ��ij�������÷������Ʊ�K2SO4����������:

���� | KCl | K2SO4 | NH4Cl | (NH4)2SO4 |
�ܽ��/g(20��C) | 34.2 | 11.1 | 37.2 | 75.4 |
��1�������Ͻ�CaCO3�гɷ�ĩ��Ŀ����________________________________��
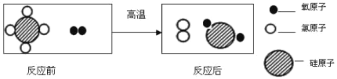
��2�����������У������ۺ�����CO2�⣬����ѭ��ʹ�õ�������______(�ѧʽ)��
��3��д����Ӧ�ڵĻ�ѧ����ʽ:___��
��4����Ӧ����������ʵ��ܽ�������ʾ����Ӧ���ڳ�������ʵ�ֵ�ԭ����____��
��5��ϴ�ӷ�Ӧ�����þ��岻��ˮ���ñ���K2SO4��Һ��Ŀ����_______������˾����Ƿ�ϴ���ķ�����_____��
����Ŀ���±����Ȼ�粒����ڲ�ͬ�¶��µ��ܽ��
�¶�/�� | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
�ܽ��/g | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.2 | 60.2 | 65.6 |
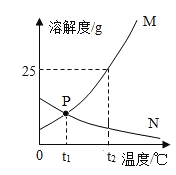
����100 gˮ�в��ϼ����Ȼ�粒����ı��¶ȣ��õ���ͼ��Ӧ����ҺA~E��
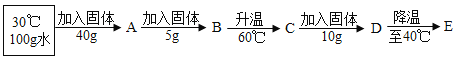
��ҺD��_____ (� ���͡������͡�)��Һ����ҺE����������__g��
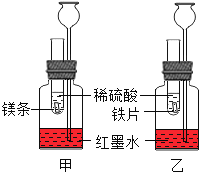
�ڽ�ʢ����ҺE��С�ձ�����ʢ��ˮ�Ĵ��ձ��У�����ձ��ڼ���NaOH���壬С�ձ��ڵĹ����ܽ⣬ԭ����______��