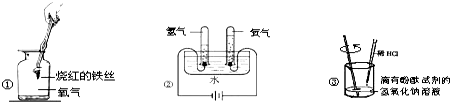
��Ŀ����
�о���ѧϰС�����ⶨijͭ���м�ʽ̼��ͭ[Cu2��OH��2CO3]�������������ֳ�ȡ15g��ͭ����Ʒ�����ձ��У�����μ���ϡ������ǡ����ȫ��Ӧ��Cu2��OH��2CO3+4HCl=2CuCl2+CO2��+3H2O��������ͭ���е����ʲ���ϡ���ᷴӦ��Ҳ������ˮ�����Ƶ��ձ���ʣ�����ʵ�����Ϊ85.8g������
��1��ͭ���м�ʽ̼��ͭ������������
��2��������Һ�����ʵ�����������
��1��ͭ���м�ʽ̼��ͭ������������
��2��������Һ�����ʵ�����������
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���,�й��������������ļ���
ר�⣺�������������뻯ѧ����ʽ���ϵļ���
��������1�������������̼�����������Ȼ��д����ѧ����ʽ�����������̼����������������ʽ̼��ͭ��������������ͭ���м�ʽ̼��ͭ������������
��2�����ݻ�ѧ����ʽ������Ȼ�ͭ��������Ȼ��������Ӧ����Һ�����������������Һ�����ʵ�����������
��2�����ݻ�ѧ����ʽ������Ȼ�ͭ��������Ȼ��������Ӧ����Һ�����������������Һ�����ʵ�����������
����⣺
�����⣬����CO2������Ϊ��73g+15g-85.8g=2.2g
��ͭ���м�ʽ̼��ͭ������Ϊx�������Ȼ�ͭ����Ϊy
Cu2��OH��2CO3+4HCl=2CuCl2+CO2��+3H2O
222 270 44
X y 2.2g
=
x=11.1g
=
y=13.5g
��1��ͭ���м�ʽ̼��ͭ����������Ϊ��
��100%=74%
��2��������Һ�����ʵ���������Ϊ��
��100%=16.5%
�𰸣�
��1��ͭ���м�ʽ̼��ͭ����������Ϊ74%
��2��������Һ�����ʵ���������Ϊ16.5%
�����⣬����CO2������Ϊ��73g+15g-85.8g=2.2g
��ͭ���м�ʽ̼��ͭ������Ϊx�������Ȼ�ͭ����Ϊy
Cu2��OH��2CO3+4HCl=2CuCl2+CO2��+3H2O
222 270 44
X y 2.2g
| 222 |
| 44 |
| x |
| 2.2g |
x=11.1g
| 270 |
| 44 |
| y |
| 2.2g |
y=13.5g
��1��ͭ���м�ʽ̼��ͭ����������Ϊ��
| 11.1g |
| 15g |
��2��������Һ�����ʵ���������Ϊ��
| 13.5g |
| 85.5g+(15g-11.1g) |
�𰸣�
��1��ͭ���м�ʽ̼��ͭ����������Ϊ74%
��2��������Һ�����ʵ���������Ϊ16.5%
���������ջ�ѧ����ʽ�ļ����ʽ�淶�ԣ�������״��㣺��Ӧ����Һ������Ҫ��ȥ��ʯ�����ʣ�

��ϰ��ϵ�д�
�����Ŀ
�����������г����ļ������ʣ�����������������ǣ�������
| A������ |
| B��̼��� |
| C�����ǣ�C12H22O11�� |
| D��������̼ |
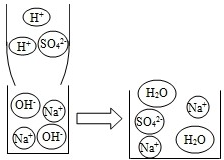 ����������һ����Ҫ�Ļ���ԭ�ϣ�ij��ѧ��ȤС������Ľṹ���Ʊ������������Լ��仯���ɵȽǶȽ����о��������Ҫ��ش���Ӧ���⣮
����������һ����Ҫ�Ļ���ԭ�ϣ�ij��ѧ��ȤС������Ľṹ���Ʊ������������Լ��仯���ɵȽǶȽ����о��������Ҫ��ش���Ӧ���⣮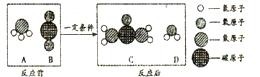 ���ʶ������ʳ����������Ҫ�����ã�����[CO��NH2��2]��һ�ֳ����Ļ��ʣ���ҵ���������صķ�Ӧ����ʾ��ͼ��ͼ��
���ʶ������ʳ����������Ҫ�����ã�����[CO��NH2��2]��һ�ֳ����Ļ��ʣ���ҵ���������صķ�Ӧ����ʾ��ͼ��ͼ��