��Ŀ����
����Ŀ��ijѧϰС��������������ijɷֽ���̽��
��֪ʶ�عˣ�
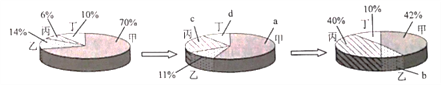
��1���������������������һ����Ҫ��;������Ŀ������������������ԼΪ_________��
��2�������ڿ�����ȼ�յķ��ű���ʽΪ___________��
�����Բ�����
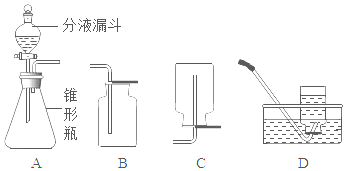
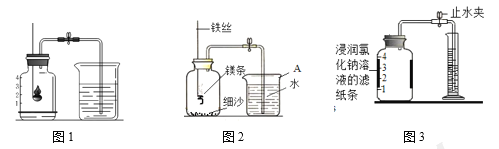
��1�����װ�������ԡ���ҽ����Һ����װ��VmL�������塣��ͼһ��ʾ������ƿ��װ��VmL����NaOH��Һ�Գ������CO2������ȼ�ճ���װ���������ף�ͨ�������£�������NaOH��Һ����Ӧ�������ӳ���ͼ����ʾװ�ã���������ʵ�װ����______����ͼһ��ѡ��

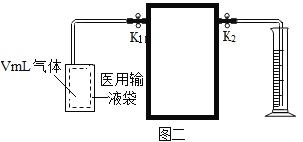
��2�����ɼ�K1��K2�����������е�����ȫ���ų���������Ͳ��Һ�����ΪV1mL��
��3���رյ��ɼ�K1��K2���ü����������ף�������ȼ�ա���װ�ú�����______����K2��������Ͳ��Һ�����ΪV2mL��
��4������������������У���������������ɱ�ʾΪ_______��100����������![]() ��100������_________���������������Ͽ�֤���������ò�����CO2
��100������_________���������������Ͽ�֤���������ò�����CO2
�����ܲⶨ��
��ͼ����ʾ����O2��CO2��ʪ�ȣ���ˮ������������̽ͷ���������������ı��ʴ��У���������ʼ�ɼ����ݣ�Ȼ������ں������塣�ɼ�������ʳƷ��ͼ����ʾ���������Ϊij��������������������������Ϊʱ�䣨S������ͼ�ش�
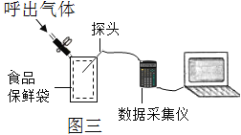
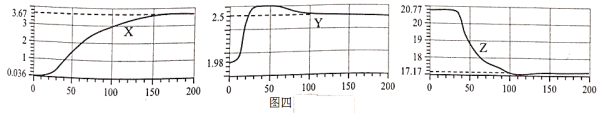
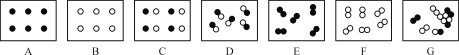
��1����ʾO2��������仯��������__________��ѡ�X������Y������Z������
��2��ʵ���У�200sʱû�вɼ����ݵ�������������������Ϊ___________��
��3��Y����60s֮�����½������ƲⱣ�ʴ��п��ܳ��ֵ�������____________��
����˼��չ��
��1��С��ͬѧ����Ϊ�����ɷ��С�������������ʡ���Ҫ����ˮ�����ȣ���ʵ����жϸ���ʶ�Ǵ���ģ�������_____________��
��2��Ϊ���ܱ������˷��еķɴ������ڿ����ɷֵ��ȶ�����ѧ������ķ����ǣ����ϰ������ڿ���ͨ��ʢ�й������Ƶ����������Ѵ�������������³������ա����й������Ƶ�������_____��
���𰸡�21% P+O2![]() P2O5 D ��ȴ������
P2O5 D ��ȴ������ ![]() >0.03% Z 76.66% ���ʴ��ڱ���ˮ�� ��ʵ���֪��������ˮ���������Ϊ1.98%���������ɷ�����������������������ֻ��0.03% ���ն�����̼��ˮ����������
>0.03% Z 76.66% ���ʴ��ڱ���ˮ�� ��ʵ���֪��������ˮ���������Ϊ1.98%���������ɷ�����������������������ֻ��0.03% ���ն�����̼��ˮ����������
��������
[֪ʶ�ع�]��1���������������������ԼΪ21%��
��2�������ڿ�����ȼ���������������ף���Ӧ�ķ��ű���ʽΪP+O2![]() P2O5
P2O5
[���Բ���]
��1������ͼ��װ�õ��ص㣬���õ��ɼ�K1��K2���������������룬������ѹǿԭ���ų�ˮ���������������������Ӧѡ��ͼһ�е�װ��D��
��3���رյ��ɼ�K1��K2���ü����������ף�������ȼ�շų��������ȣ���װ�ú�������ȴ���������ٴ�K2��������Ͳ��Һ�����ΪV2mL��
��4������������������У���������������ɱ�ʾΪ![]() ��100%�������ɷֵĶ�����̼�������Ϊ 0.03%���������������������Ϊ 0.03%��������
��100%�������ɷֵĶ�����̼�������Ϊ 0.03%���������������������Ϊ 0.03%��������![]() ��100������0.03%���������Ͽ�֤���������ò�����CO2��
��100������0.03%���������Ͽ�֤���������ò�����CO2��
[���ܲⶨ]��1����������������̼��ˮ��������ʪ�ȣ�̽ͷ������У���ʼ�ɼ����ݣ�Ȼ������ڴ������壬�ɼ������ݾ����������ں��������ж�����̼�����ˮ�������࣬�������٣���ʾ������������仯����Z��Y�����������60s����С��ԭ����ˮ����������
��2����ͼʾ��֪��200sʱδ�ɼ����ݵ��������壬���������Ϊ76.66%��
��3��Y�����������60s����С��ԭ����ˮ����������
[��˼��չ]
��1����ʵ���֪��������ˮ���������Ϊ 1.98%���������ɷֵ����������������Ϊ 0.03%������Ϊ�п����ɷֵ��������������������Ҫ��ָˮ������˵���Ǵ������
��2��Ϊ���ܱ��ַɴ������ڿ����ɷֵ��ȶ������ϰ������ڵĿ���ͨ��ʢ�й������Ƶ���������ʱ�Ա�����Ķ�����̼��ˮ������������Ʒ�Ӧ����������������

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�����Ŀ����ѧʵ���г�����������������������������ѧ�����������û��ᣬҪ���ݾ����������Դ������ڷ��ֺͽ�����⣮���磺С�ͬѧ�ڿ����е�ȼþ��ʱ��ȴ���������ɵİ�ɫ�����л������������ĵ���ɫ���塣
��������⣩Ϊʲô�����ɵ���ɫ���壿
���������ϣ�С溲������ϣ���¼�����м������ʵ���ɫ��
�� �� | MgO | MgCl2 | Mg3N2 | Mg(NO3)2 | MgCO3 | Mg(OH)2 |
�� ɫ | ��ɫ | ��ɫ | ����ɫ | ��ɫ | ��ɫ | ��ɫ |
����ͬѧ��Ϊ���ز����Ȼ�þ����ɫ��������________��
��������룩�������ϣ�С���Ϊ����ɫ�����������þ������е�________���ѧʽ����Ӧ���ɵģ�
��ʵ��̽����С����ʵ��֤ʵ���Լ��IJ��룬����ʵ�����������_________��
��ʵ����ۣ�����С�ʵ������д��þ���ڿ�����ȼ��ʱ������Ӧ���ű���ʽ��__________��______________��
����˼�����ۣ�ͨ������ʵ�飬���ȼ����ʲô�µ���ʶ��__________��
����Ŀ�����������������ⶨ������ʶ
��. �ú��ײⶨ�����������ĺ�����
��1����д�������ڿ�����ȼ�յĻ�ѧ����ʽ________��
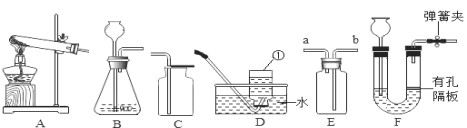
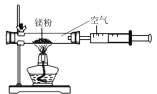
��2����ͼ1��ʾ����ʵ�飬 ��ÿ����������ĺ���С��1�M5�����ܵ�ԭ���� ____������ţ���
A��ȼ�ճ����뼯��ƿ̫�� B������������
C�� ʵ���з��ֵ��ܿ�������ð�� D��װ��©��
��. ��þ���ⶨ�����������ĺ�����
��3����д��þ����������ȼ�յĻ�ѧ����ʽ_____________��
��4��ijͬѧ����ͼ2ʵ��ⶨ��������������ʱ���֣������л�����������ɫ���塣
��֪��þ���뵪����Ӧ���ɵ���ɫ�ĵ���þ��Mg3N2�����塣 ���ڿ�����ȼ��þ��ʱ���ѹ۲쵽���������е���ɫ���壬ԭ����_________���ڲ����淶������£���ͬѧʵ������õ������������______1/5������ڡ�����С�ڡ����ڡ�����
��. �����۲ⶨ�����������ĺ�����
��5����֪��������������е�������ˮ��Ӧ�������⣨��Ҫ�ɷ���Fe2O3�qxH2O������д���÷�Ӧ�Ļ�ѧ����ʽ________��
��6��ijͬѧ�����������ԭ���������۲ⶨ�����������ĺ������������ͼʵ�飬8���Ӻ������������
ʵ��ǰ����� | ʵ������� | |
����ƿ�ڿ��� | ��Ͳ��ˮ | ��Ͳ��ʣ��ˮ |
250mL | 180.0mL | 129.5mL |
ʵ��ʱ��ͬѧ���ǽ����۶���ƿ�ף����ǽ��������ڽ������Ȼ�����Һ����ֽ���ϣ��ٰѸ���ֽ�����ڹ��ƿ�ڲ࣬��Ŀ����________�����ݱ������ݼ���ó��������������������Ϊ________�����������0.1%�������ú���ȼ�յķ�����ȣ��ø÷����ⶨ�����������ĺ�������Ҫ�ŵ�һ��û����Ⱦ������ ____________��
��.��չӦ��
��7��ȡ2.4gþ����O2��N2�Ļ����������ȫȼ�գ����ù�������Ϊag,����a��ȡֵ��Χ��________g��a g��4g�����������0. 1g����
��8����֪��Fe2O3������һ���ĸ������ֽܷ�Ϊһ�ָ��ӵ������������һ�����塣 �����������___________�����顣�ֽ�64.0gFe2O3������ȵ����¶��£����ֹ�������������3.2g���������ٸı䡣��ش�64.0gFe2O3�����к�����Ԫ��_______g�����¶��¸��ӵ�����������Ļ�ѧʽΪ__________��