��Ŀ����
��9�֣���������ۺ����ۡ�����ۡ��۸����ܴ���ۡ��ָ�û�����Բ��죬ij��ѧ��ȤС��ͬѧ�Զ��߳ɷֵIJ�ͬ������̽����
���������ϡ�
�����������������ӹ����ɣ�����һ������̼��ơ�������ȣ�����������ܽ���ˮ������Ũ���Ტ���Ȼ���ֻ�ɫ���ɫ��
�����ۡ�����ۡ��ɱ��Ǽӹ����ɣ��ӹ������л��õ��������ƣ����ǵ���Ҫ�ɷ���̼��ơ�
����������ۺ����ۡ�����ۡ��У���̼����⣬�����ɷ־����������ᷴӦ���ɶ�����̼��
��������롿��������ۺ����ۡ�����ۡ�����������У�
�����ۡ�����ۡ��к��� ��
�����ۡ�����ۡ��в��������ᡣ
����������ۺ����ۡ�����ۡ���̼��Ƶ�����������ͬ��
��ʵ���������������ۺ����ۡ�����ۡ��ֱ������ֻ�ձ��У�������ˮ�ܽ⣬����һ��ʱ�����ˣ�ȡ������Һ���á�
| ���� | ���� | ���� |
| ȡ����������Һ�ֱ�����֧�Թ��У��μ����� | ��������۵���Һû�б�ɫ�����ۡ�����ۡ�����Һ��� | ����ٳ��� |
| ȡ����������Һ�ֱ�����֧�Թ��У�����һ���� ������������ | ��������۵���Һ�л�ɫ���֣��ֲ���ڣ����ۡ�����ۡ�����Һû���������� | ����ڳ��� |
| | ��������� | ���ۡ�����ۡ� |
| �����ĩ������ | 10.00 g | 10.00 g |
| ����ϡ��������� | 46.00 g | 50.13 g |
| ��Ӧ���ձ������ʵ����� | 52.00 g | 55.77 g |
����д��������̣����������0.1%��
�ó����ۣ����ۡ�����ۡ���̼��Ƶ��������� ��������ۣ�ѡ����ڡ��������ڡ����ڡ�����
����˼�����ۡ���֪ʵ���������ϡ�������������Ϊ14.6% ��С��������ü���ϡ�����������������������������μӷ�Ӧ���Ȼ�����������������û�ѧ����ʽҲ��������ۡ�����ۡ���̼��Ƶ�����������ΪС����˼·�Ƿ���У� ��ѡ����С������С�������������� ��
����������������ۡ�����ۡ��ɱ��Ǽӹ����ɣ��ӹ������л��õ��������ƣ��������ۡ�����ۡ��к����������ƣ����ۡ�����ۡ��к����������ƣ��������Ƴʼ��ԣ�����Ӧ������Һ�м����̪��Һ����Һ�ʼ��ԣ���̪���죬��������۵���Һ���а����ᣬ���������Ե����ʣ�����Ӧ�������ᣬijЩ�����������������ɫ���ɼ���ɵó����ۡ�����ۡ���̼��Ƶ���������������������ۣ�������������л����������������ƣ����Լ����ϡ������Ⱥ��������Ʒ�Ӧ�����¼���Ľ������
���㣺ʵ��̽����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���11�֣������װ�г�ʹ��һ�ִ�װ��������Ʒ��Ϊ��504˫�����������ǩ����ͼ��ʾ��ͬѧ�Ƕ�һ�����õġ�504˫������������Ʒ�ܺ��棬���ʵ�����̽����
��������⡿���ù���ijɷ���ʲô��
���������ϡ������Ȼ�����Һ�ڳ����·�����Ӧ�����Ȼ�������
���������롿���ù����п��ܺ���Fe��Fe2O3��CaO��Ca(OH)2��CaCO3��
���ù����п��ܺ���Ca(OH)2��ԭ����(�û�ѧ����ʽ��ʾ) ��
��ʵ��̽��1��
��ͬѧ�ķ�����
| ʵ����� | ʵ������ | ʵ����� |
| ��ȡ������������Թ��У�����������ˮ�ܽ⣬���ú�ȡ�ϲ���Һ�μ���ɫ��̪��Һ�� | �����ܽ�ʱ�Թ���ڷ��̣��Թܵײ��в������Һ��졣 | ������һ������ �� ���������ơ� |
| ����ȡ������������Թ��У��μ�����ϡ���ᡣ | ��������ʧ���д�����ɫ����������õ�dz��ɫ��Һ�� | ������һ������ �� �� һ������Fe2O3�� |
| �ǽ�������в���������ͨ�뵽�����ʯ��ˮ�С� | �� | ������һ������CaCO3�� |
����ͬѧ��Ϊ��ͬѧ��ʵ���в����ܵó�һ����Ca(OH)2�Ľ��ۣ�������
��
�Ʊ�ͬѧ��Ϊ��ͬѧ��ʵ�鲢���ܵó�һ������Fe2O3�Ľ��ۣ�������(�û�ѧ����ʽ��ʾ) �� ��
��ʵ��̽��2��
�ҡ���ͬѧ�������ʵ�鷽��������֤��

�� �ҡ���ͬѧʵ�������ܵó�������Ʒ��һ�������� ��
�� ��ͬѧ����ʵ������������C��CaCO3 ��������Ϊ1.0 g��������ҺA�к�����
���Ƶ���������д��������̣�
�� ��ͬѧ����ʵ�����������������к������ʵ�������Ϊ1.6 g������B��CaCO3������Ϊ1.0 g��
��ʵ����ۡ��ۺ�����ʵ���Լ��ҡ�����λͬѧ��ʵ�����ݣ����ù���ijɷ��ǣ�
��7�֣���һ����ɫ������Ʒ��������̼���ơ����ᱵ���������ơ��Ȼ����е�һ�ֻ��֡�Ϊ̽����ɷ֣�С����ʦȡ��һ������Ʒ��������ˮ�ܽ⣬���˵õ���ɫ��������ɫ��Һ�ҡ�
д�����������п��ܷ�����Ӧ�Ļ�ѧ����ʽ�� ��
����ʱ�õ����������������������� ��
��̽���һ�� �ܽ�С��̽����ɫ�����ijɷ֡�
| ʵ����� | ʵ������ | �� �� |
| ȡ��ɫ�����ף��μ�������ϡ���� | ����������ʧ | ��ɫ��������һ������ |
| ʵ����� | ʵ������ | ���� | |
| ʵ��� | ȡ������ɫ��Һ�ң�����̼������Һ | ���������� | ��ɫ��Һ����һ������ |
| ʵ��� | ����٣�ȡ������ɫ��Һ�ң���������� ��Һ������ | ���ɰ�ɫ���� | ԭ��ɫ������Ʒ��һ�����ڸ����� |
| ����ڣ�ȡ������е���Һ���μ� ��Һ | | ||
�����ۡ� ͨ������С��Ĺ�ͬ̽�����ó���ԭ��ɫ������Ʒ���еijɷ֡�
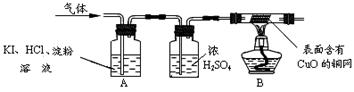
��18�֣���ҵ�ռNaOH�����нϺõ�ɱ�����������������ã�����ҵ�ռ��г���������̼���ơ�ij��ѧѧϰС��ͬѧΧ�ƹ�ҵ�ռ�չ��ϵ���о���
̽��һ����ҵ�ռ����Ƿ���̼����
���������ϡ�̼���ƺ��Ȼ����ܷ������ֽⷴӦ��
С��ָ����ѡ������ʵ��ҩƷ��̽����ϡ���ᡢ��̪��Һ��CaCl2��Һ��
��1��С��ͬѧ�������ۺ�һ����Ϊʹ�� ҩƷ���ܴﵽ̽��Ŀ�ģ������� ��
��2�������ѡ�õ�ʵ��ҩƷ�������ʵ�鱨�棺
| ʵ����� | ʵ������ | ʵ����� |
| ȡ������ҵ�ռ���Ʒ�����Һ�μӹ��� | | ��ҵ�ռ��к���̼���ƣ�����ʵ������Ļ�ѧ����ʽ�� �� |
С��ͬѧ������ͼ��ʾװ�òⶨ��ҵ�ռ���Ʒ�Ĵ��ȣ��̶�װ��ʡ�ԣ�

��ʵ�鲽�衿
�ٰ�ͼ����װ�ã������װ�õ������ԣ�
�ڳ���һ�������ռ���Ʒ�����C��������
�۴��ɼ�K������N2һ��ʱ�䣻
�ܽ���װ��C��D���رյ��ɼ�K��װ��A�м���ϡ���ᣬ�����ٲ�������Ϊֹ��
���ظ�����۲������������C�����������ӡ�
�Իش�����������⣺
��1��װ��B��Ũ����������� ����֪��ʯ�ҵ���Ҫ�ɷ���CaO��NaOH����װ��D�������� ��
��2������۲�����Ŀ���� ����������û���ظ�����۵IJ�������ⶨ�ռ���Ʒ�Ĵ��Ƚ� ��ѡ�ƫ�ߡ�����ƫ�͡�����Ӱ�족����
��3��������и����C�����������ӣ�˵�� ��д��NaOH�����ɵ����巴Ӧ�Ļ�ѧ����ʽ�� ��
��4�����в�����Ӱ�쵽����������� ������ţ���
A����ϡ�����Ϊϡ���� B��ϡ�������
C������N2ʱ��ϳ� D��ʡ��װ��D
��8�֣���1��С������ͼ��ʾװ�öԶ�����̼�����ʵ������о���
�۲쵽��������____________________________________��
ʵ��Ľ�����______________________________________��
��2����������⡿������̼�ܷ�֧��ȼ�գ�
��������衿þ���ڶ�����̼��ȼ�ա�
���������ϡ�������þ��������þ���ǰ�ɫ������ˮ�Ĺ��塣
��MgO+2HCl= MgCl2+H2O ��
��MgCl2+2NaOH=Mg(OH)2��+2NaCl ��
��ʵ��̽����
����ͼ��þ������ȼ�գ�ð���̣��к�ɫ�������ɣ����ų��������ȡ�
��Ϊ����������ijɷ֣�������ʵ�顣
| ʵ�鲽�� | ʵ������ | ʵ����ۺͻ�ѧ����ʽ | |
| ����ƿ�м���������ᣬ��ַ�Ӧ����ˣ�����ֽ�����к�ɫ���塣 | I������ɫ�����ռ���ϴ�ӡ������ȼ���ڻ����Ϸ���һ��պ�г���ʯ��ˮ���ձ��� | ��ɫ����ȼ�գ��ձ��ڱڳ��ְ�ɫ���ǡ� | ��ɫ������______�� ��Ӧ�Ļ�ѧ����ʽ�ǣ� ___________________�� |
| II��ȡ������Һ���Թ��У���μ�������������Һ�� | ��ʼ_______�����а�ɫ���������� | ���̵ijɷ���______�� | |
��Ϊ�˻���II�еİ�ɫ������֤��������ȫ��ʵ�鷽����______________________
___________________________________��Ҫ��д�����衢�Լ�������ͽ��ۣ���
����˼��ߡ�ʵ��������ijЩ���ý���������Ż𣬲����ö�����̼���Ӧ��ϸɳ���