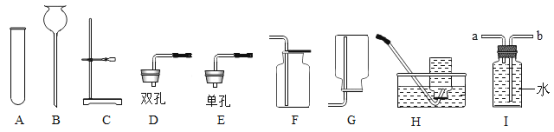
��Ŀ����
����Ŀ����ͼ���ճ������г��õĴ���ʾ��ͼ�� ��ش��������⣺
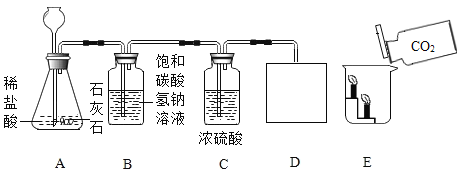
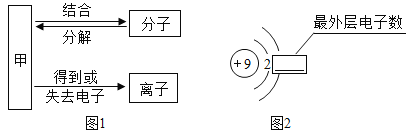
��1��ͼʾ�����ڽ������ϵ���______������ţ���ͬ���������л��ϳɲ��ϵ���________��
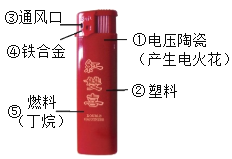
��2������ȼ�ϵ���Ҫ���Ƕ��飨C4H10����д������ȼ�յĻ�ѧ����ʽ_______________��
��3��������ͨ��״���³���̬�� �д̼�����ζ������ż��δ����ʱ���ŵ�һ�ִ̼�����ζ�� ˵��_____________________���ӷ��ӵĽǶȷ���)��
��4������ʹ�ý���ʱ�ɿ�ȼ�Ϸ��� �����ͻ�Ϩ�� ��������ݵ�ԭ����_____��
���𰸡��� �� 2C4H10 + 13O2![]() 8CO2 +10 H2O �����ڲ����˶� �����ȼ��
8CO2 +10 H2O �����ڲ����˶� �����ȼ��
��������
��1��ͼʾ�����Ͻ����ڽ������ϣ�����ܣ����������л��ϳɲ��ϣ�����ڡ�
��2������ȼ�����ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��2C4H10 + 13O2![]() 8CO2 +10 H2O��
8CO2 +10 H2O��
��3��������ͨ��״���³���̬�� �д̼�����ζ������ż��δ����ʱ���ŵ�һ�ִ̼�����ζ�� ˵���������ڲ����˶���
��4������ʹ�ý���ʱ�ɿ�ȼ�Ϸ��� �����ͻ�Ϩ�� ��������ݵ�ԭ���ǣ������ȼ�

 ��ڽ��ȫ������ϵ�д�
��ڽ��ȫ������ϵ�д�����Ŀ����ҵ���Ըߴ��ȵĶ�������Ϊԭ���Ʊ�������ص���Ҫ�������£�

��֪������ʵ��ܽ�ȣ�20�棩���±���
���� | K2CO3 | K2SO4 | KMnO4 |
�ܽ��/g | 111 | 11.1 | 6.34 |
��1��KMnO4����Ԫ�صĻ��ϼ�Ϊ_____��
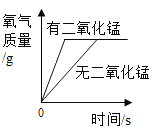
��2������I��������_____�����������������¶Ȳ��˹��ߵ�ԭ����_____���û�ѧ����ʽ��ʾ����
��3����ͨCO2�ữ������Ӧ�Ļ�ѧ����ʽΪ��3K2 MnO4+2CO2�T2KMnO4+MnO2��+2K2CO3������ϡH2SO4�ữ����Ӧ�Ļ�ѧ����ʽΪ��3K2 MNO4+2H2SO4�T2KMnO4+MnO2��+2K2SO4+2H2O����ҵ�ϲ�����ϡH2SO4�ữ��ԭ����_____��
��4����ⷨҲ����ʵ����K2MnO4��KMnO4��ת������Ӧ�Ļ�ѧ����ʽΪ��2K2 MnO4+2H2O![]() 2KMnO4+2KOH+H2��������ͨCO2�ữ����ȣ����ŵ���_____��
2KMnO4+2KOH+H2��������ͨCO2�ữ����ȣ����ŵ���_____��