��Ŀ����
����Ŀ����ҵ������������ Al2O3 �� SiO2���������������ʣ���ȡ�������Ļ�����������ͼ��ʾ��
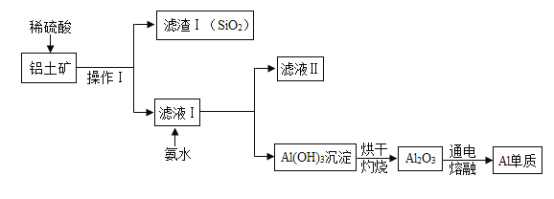
��1��ͨ�������̿ɵó�SiO2��������_________������ţ���
A������ˮ B������ϡ���ᷴӦ C��������������Һ��Ӧ
��2������������������ϡ���ᷴӦ������������ˮ�Ļ�ѧ����ʽΪ_______________��
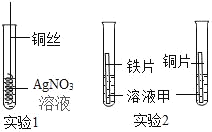
��3������ I ������Ϊ___________��
��4�����Ͻ���Ͼ��нϺõĿ���ʴ�ԣ���Ӧ�Ļ�ѧ����ʽΪ___________________��
���𰸡�AB Al2O3+3H2SO4=Al2(SO4)3+3H2O ���� 4Al+3O2=2Al2O3
��������
��1��ͨ�������̼���ϡ��������ܵõ�����������壨���������ɵó�SiO2������ˮ�Ҳ�����ϡ���ᷴӦ�����ʣ���ѡAB��
��2������������������ϡ���ᷴӦ������������ˮ����صĻ�ѧ����ʽΪAl2O3+3H2SO4=Al2(SO4)3+3H2O�����Al2O3+3H2SO4=Al2(SO4)3+3H2O��
��3������I������Ϊ���ˣ���Ϊ�����˲�����������Һ������˷��룬������ˡ�
��4�����Ͻ���Ͼ��нϺõĿ���ʴ�ԣ�����Ϊ�������ڿ�������������Ӧ�������ܵ���������Ĥ���䷴Ӧ�Ļ�ѧ����ʽΪ��4Al+3O2=2Al2O3�����4Al+3O2=2Al2O3��

 ��У����ϵ�д�
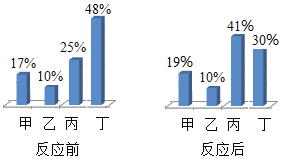
��У����ϵ�д�����Ŀ�����и�ѡ������ͼ��ʾ����������ǣ�������
ѡ�� | ������ | ������ |
A | ��һ����ϡ�����м���п�۵����� | ��Һ����Ԫ�ص����� |
B | ��һ�������������ƺ�̼���ƻ����Һ�м���ϡ��������� | ������������� |
C | һ����������ͭ��Һ�м����Ȼ������ɳ��������� | ���ɳ��������� |
D | ��һ���¶��£���һ�����ı����������Һ�м�������ع�������� | ��Һ�����ʵ��������� |
A.AB.BC.CD.D