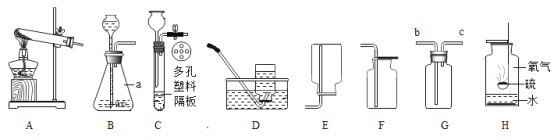
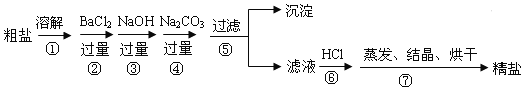
��Ŀ����
����Ŀ��ijУ��ѧС�����ˮ���ײ�ˮ������Ҫ�ɷֽ���������̽������������»���ش�������⡣
���������ϣ���Ȼˮ�к���Ca2+��Mg2+��HCO3-�����ӣ��ڼ��������£���Щ�������������ܽ�ȸ�С��������ˮ������Ҫ�ɷ�Ϊ̼���κͼ�й����ʵ��ܽ��Լ��±���
������ ������ | OH- | CO32- | HCO3- |
Ca2+ | �� | ���� | ���� |
Mg2+ | ���� | �� | ���� |
��������룩ˮ������Ҫ�ɷ�һ����CaCO3��_____���ѧʽ�������ܺ���Ca(OH)2��______��
��������⣩ˮ������Ҫ�ɷ����Ƿ���Ca(OH)2��MgCO3�أ�
����Ʒ���1��ȷ��ˮ�����Ƿ�Ca(OH)2
ʵ�鲽�� | ʵ������ | ���� |
�����������ˮ���У���������������ˮ��ֽ��裬���ˣ�����Һ�����Na2CO3��Һ�� | ______________ | һ������Ca(OH)2 |
��ʵ�鷽��2��ȷ��ˮ�����Ƿ�MgCO3
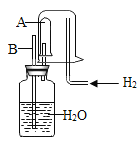
��������ʵ��װ�ã����ʵ��2̽��������Ҫʵ�鲽�����£�

����Ҫʵ�鲽�����£�����D��Eװ��������Ϊ200.0g������ͼ��װ��2.5gˮ������������ƿ�У���������ϡ������Һ������ƿ�в��ٲ�������ʱ����ֹˮ��K���ӵ���a���������������һ��ʱ������װ��D��E��������Ϊ201.25g����ʯ����Ҫ�ɷ�ΪCaO��NaOH��������ϡ����Ļӷ����Լ�װ���ڿ�������������ʵ���Ӱ�죩��
��ʵ�����ۣ�
��1����Ӧ�������ֹˮ��K���������������Ŀ����_____A��Bװ��֮��_____���ø���װ�á�������Ҫ��������Ҫ������������______������������
��2��װ��Bʢ��ϡ��������������ǣ�______
��3��װ�� B����������ķ�Ӧ�Ļ�ѧ����ʽΪ______����������
��4��װ��B���������������Ϊ______gͨ������˵����ˮ����____������ţ���MgCO3��
A һ�� B һ���� C ���� D ��ȷ��
��֪CaCO3��Է�������Ϊ100.Mg CO3��Է�������Ϊ84����������������
��ʵ�鷽��3��ȷ��ˮ�����Ƿ���Mg(OH)2
��5����ȡ����Ϊ1.2g��ˮ������������3.65%ϡ������֮ǡ����ȫ��Ӧ���������ϡ�����g��������ʵ�飬�жϵ�����ֵ����_____����ʱ��ˮ����һ������Mg(OH)2
���𰸡�Mg(OH)2 MgCO3 û�а�ɫ�������� ʹ���ɶ�����̼��������� ��Ҫ ����Ӱ�����ն�����̼���� ��������ĵμ����ʣ�ʹ��Ӧ��ֽ��� CaCO3+2HCl==CaCl2+H2O+CO2�� 1.25 A ������28.6g
��������
[�������] ��Ȼˮ�к���Ca2+��Mg2+��HCO3-�����ӣ��ڼ��������£���Щ�������������ܽ�ȸ�С�����ʣ�����ܽ��ԣ�ˮ������Ҫ�ɷ�һ����CaCO3��Mg(OH)2�����ܺ���Ca(OH)2��MgCO3��
[��Ʒ���1] ������������̼���Ʒ�Ӧ����̼��Ƴ����������������ˮ���У���������������ˮ��ֽ��裬���ˣ�����Һ�����Na2CO3��Һ��û�а�ɫ�������ɣ�ˮ����һ������Ca(OH)2��
[ʵ�鷽��2]
��1����Ӧ������,��װ��B�в���������Ϊ������̼����ֹˮ��K���������������Ŀ�����ž�װ�õĶ�����̼��ʹ������̼��������գ�A��Bװ��֮�䲻Ҫ���ø���װ�á��������Ǿ�������������Һ����������н϶��ˮ����������ˮ�����ܱ�C��Ũ�������գ����ᱻ����������Һ���գ���Ӱ�������̼��������
��2��װ��Bʢ��ϡ��������������ǣ���������ĵμ����ʣ�ʹ��Ӧ��ֽ��У�
��3��װ�� B����������ķ�Ӧ��̼��ƺ����ᷴӦ���ɶ�����̼��ˮ���Ȼ��ƣ���ѧ����ʽΪCaCO3+2HCl==CaCl2+H2O+CO2����
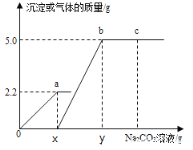
��4��װ��D��E�������ն�����̼����ӦǰD��Eװ��������Ϊ200.0g��һ��ʱ������װ��D��E��������Ϊ201.25g��˵�����ɶ�����̼1.25g����2.5g̼�����ȫ��Ӧ���ɶ�����̼������Ϊx��2.5g̼��þ��ȫ��Ӧ���ɶ�����̼������Ϊy��
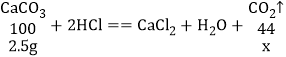
![]() x=1.1g
x=1.1g
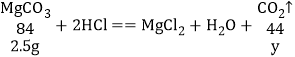
![]() y>1.1g ̼��þ��ͬ������̼������ɶ�����̼�ࣻ
y>1.1g ̼��þ��ͬ������̼������ɶ�����̼�ࣻ
����ˮ����ͬ������̼������ɶ�����̼�࣬��֪ˮ����һ������̼��þ��
��5����ȡ����Ϊ1.2g��ˮ������������3.65%ϡ������֮ǡ����ȫ��Ӧ���������ϡ�����g����1.2g̼����������������Ϊm��1.2g̼��þ�������������Ϊn��1.2g������þ�������������Ϊz��
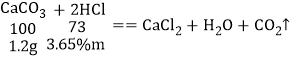
![]() m=24g
m=24g
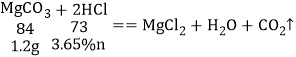
![]() n��28.6g
n��28.6g

![]() z��41.2g
z��41.2g
����������㣬������ֵ�������28.6g����ʱ��ˮ����һ������Mg(OH)2��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�