��Ŀ����
��5�֣������³��ڵ�����������Ҫ�����ĸ��������г�������Ʒ���������ʲ�����ˮҲ�����ᷴӦ����ʵ��С��ͬѧΪ�ⶨ�䴿�ȣ�
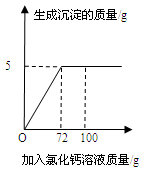
����������ʵ�飬ʵ���������±���ʾ����ش��������⣺
| | ��1�� | ��2�� | ��3�� |
| ��Ʒ������g�� | 25 | 30 | 25 |
| ����������g�� | 200 | 100 | 100 |
| ������Һ������g�� | 216 | 116 | 116 |
��2���г���2�βμӷ�Ӧ����������������(X)�ı���ʽ ��
��3����ǡ����ȫ��Ӧ�����Һ�м���46��5gˮ�����������õ���Һ�����ʵ���������Ϊ ��
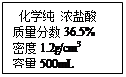
��4������������ǩ��ʾ��Ũ������������ʵ������������ᣬ����ȡŨ��������
Ϊ mL��
��5����500t�ÿ�ʯ���Ƶú����ʵ���������Ϊ ��
��1��Fe2O3+6HCl===2FeCl3+3H2O ��2��=
��3��20% ��4��200 ��5��404t
���������������1�����������Ҫ�ɷ����������������ᷴӦ�Ļ�ѧ����ʽΪ��Fe2O3 + 6HCl = 2FeCl3 + 3H2O
��2�� ��������ʵ�����ݷ��������γ�������Ʒ�е�������ǡ����100g���ᷴӦ��ȫ������������Һ����������֪25g��������Ʒ�е�������������Ϊ16g�����Ը��ݷ���ʽ��Fe2O3+6HCl==2FeCl3+3H2O��Fe2O3��HCl��������ϵ160:219�����Ե�2�βμӷ�Ӧ����������������(X)�ı���ʽ��160:219=16g��x
��3�� ������������������γ�������Ʒ�е�������ǡ����100g���ᷴӦ��ȫ���ɸ��ݷ���ʽ��Fe2O3+6HCl==2FeCl3+3H2O����������ʵ�����
�⣺��FeCl3������Ϊx
Fe2O3+6HCl==2FeCl3+3H2O
325
16g x
160��325=16g��x x=32��5g
��Һ������=116g+46��5g=162��5g
�������õ���Һ�����ʵ���������=32��5g/162��5g��100%=20%
��4��ͨ���ڶ��ʵķ�����100g��������������Ϊ21��9g, ����ʵ�������������400g������������=21��9g��4=87��6g������������Ũ��������Ϊv,����ʽΪ��V��1��2g/mL��36��5%=87��6g,V=200g
��5����Ϊ25g��������Ʒ�е�������������Ϊ16g ������500t�ÿ�ʯ��������������=320t,����������=500t-320t=180t������������=320t��112/160��100%=224t�����Ժ����ʵ���������Ϊ=180t+224t=404t
���㣺ʵ�����ݷ����ʹ��������ݻ�ѧ����ʽ����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�(7��)ˮ������֮Դ����������ϧˮ��Դ��ÿ����������Ρ�
��1����Ȼ���е�ˮ���Ǵ�ˮ�������ó����� ������������ȷ������Ծ���ˮ��
��2��ˮ���������Ƹ�����Һ��������������ˮ����Һ�¶��������ߵ��� ��
| A���������� | B������� | C������� | D��Ũ���� |
��4������ˮƿ�þú�ƿ���ڱڳ�����һ��ˮ�����Լ����ȥƿ����5g CaCO3���������躬5%�����ʳ�������Ƕ��٣�
�ۻ�ѧ����ʽΪ��CaCO3 + 2CH3COOH= (CH3COO)2 Ca + H2O + CO2����
(����������)
��ˮ����Ҫ�ɷ���CaCO3��Mg(OH)2��һЩ������������ʣ�����ȥ5g��ˮ���������ʳ����������������ݱȽϣ��� (����ӡ����ٻ�ȷ����)
����ݻ�ѧ����ʽ���㣺��ȫ�ֽ�340g������������Ϊ10%�Ĺ���������Һ�еĹ������⣨H2O2����������������������
| A��16g | B��160g | C��32g | D��1.6g |