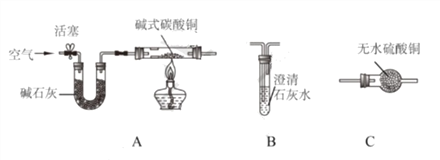
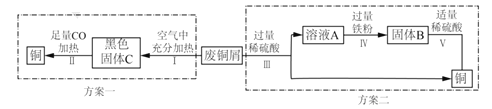
��Ŀ����
����Ŀ������ľ̿��һ����̼������ͭ����������ϡ�����������ʣ�����֮�䷢���ķ�Ӧ��������A+B��C+D����ʾ��
��1����AΪ���ʣ�A��B�ڸ����·�Ӧ���ɹ۲쵽�����ĩ�ɺ�ɫ��죬��B��_____���йط�Ӧ�Ļ�ѧ����ʽΪ_____��
��2����AΪ���廯���A��B�ڸ����·�Ӧ���ɹ۲쵽�����ĩ�ɺ�ɫ��ڣ���A��_____��B��_____��
��3����A��ҺpH��7��A��B�ڳ����·�Ӧ���ɹ۲쵽��Һ����ɫ��Ϊ��ɫ����A��B��Ӧ�Ļ�ѧ����ʽΪ_____��
���𰸡�����ͭ C+2CuO![]() 2Cu+CO2�� һ����̼ ������ 3H2SO4+Fe2O3��Fe2(SO4)3+3H2O
2Cu+CO2�� һ����̼ ������ 3H2SO4+Fe2O3��Fe2(SO4)3+3H2O
��������
�������ʵ����ʽ��з���������ͭ�Ǻ�ɫ��ĩ���ܱ�̼���»�ԭΪͭ���������Ǻ�ɫ��ĩ���ܱ�һ����̼���»�ԭΪ����pHС��7����Һ�����ԣ�������������Ӧ������ɫ��������Һ���ݴ˽��
��1����AΪ���ʣ�A��B�ڸ����·�Ӧ���ɹ۲쵽�����ĩ�ɺ�ɫ��죬����ͭ�Ǻ�ɫ��ĩ���ܱ�̼���»�ԭΪͭ�����Կ�����ɫ��ĩ��������B������ͭ������ͭ��̼��Ӧ����ͭ�Ͷ�����̼���仯ѧ����ʽΪC+2CuO![]() 2Cu+CO2����
2Cu+CO2����
��2����AΪ���廯���A��B�ڸ����·�Ӧ���ɹ۲쵽�����ĩ�ɺ�ɫ��ڣ��������Ǻ�ɫ��ĩ���ܱ�һ����̼���»�ԭΪ�����ܹ۲쵽��ɫ��ĩ��ڵ������B����������
��3����A��ҺpH��7����A����Һ��A�����ᣬ����������������Ӧ������������ˮ����������ˮ��Һ�ǻ�ɫ�ģ����3H2SO4+Fe2O3��Fe2��SO4��3+3H2O��

 ��У����ϵ�д�
��У����ϵ�д�