��Ŀ����
 ��������ͭ������㷺ʹ�õ����ֽ���������������ϢϢ��أ�ͼ1��ϡ��Ũ����ʵ���ʾ��ͼ��
��������ͭ������㷺ʹ�õ����ֽ���������������ϢϢ��أ�ͼ1��ϡ��Ũ����ʵ���ʾ��ͼ����1��д��ͼ1��a��b�������������ƣ�a
������
������
��b��Ͳ
��Ͳ
����2��b������ʢ���Լ���
Ũ����
Ũ����
���ˮ����Ũ���ᡱ������3����֪M��N�ֱ���ϡ���ᡢ����������Һ�е�һ�֣�ij��ѧ��ȤС��ͬѧ��һ������M�в��ϵμ�N�����ⶨ������Һ��pH����ͼ2��ʾ��
��M��
����������Һ
����������Һ
���÷�Ӧ�Ļ�ѧ����ʽ2NaOH+H2SO4�TNa2SO4+2H2O
2NaOH+H2SO4�TNa2SO4+2H2O
���ڷ�Ӧ�����У���ʦȡa��b��c������Ӧ�����Һ������˳�����ͬѧ�Dz��ⶨ��ҺpH��������ʵ�鷽����������Һ����̽����
С����С��ֱ�ȡ����һ����Һ����ʵ�飺С������ȡ��Һ�м���
CuSO4
CuSO4
��Һ���۲쵽����ɫ�������ɣ����ۣ���a����Һ��С����������һ����Һ�еμӷ�̪��Һ���۲쵽
��Һ����ɫ
��Һ����ɫ
���ۣ���b����c����Һ��
Ϊ��һ��ȷ���ô���Һ�ɷ֣�С������Ʋ��������ʵ�飺
| ʵ�鲽�� | ʵ������ | �� �� |
ȡ��������Һ�� ȡ��������Һ�� �����еμ� �����еμ� ʯ�� ʯ�� ��Һ ��Һ |
��Һ��� ��Һ��� |
Ϊc����Һ������֪��Һ�е������� Na+��H+��SO42- Na+��H+��SO42- �������ӷ��ţ� |
��������1������ʵ���ҳ������������ƺ���;��գ�
��2������Ũ����������Լ�Ũ����ϡ����ע�����������գ�
��3���ٸ������ߵ�������7�������ж�����ҺΪ������Һ�����ݷ�Ӧԭ��д���÷�Ӧ�Ļ�ѧ����ʽ��
��С�����������֪����ɫ������������ͭ���ǿ����Ե�ͭ�����������Ʒ�Ӧ���ɵģ��ɴ˿����Ƴ�ԭ��Һ����ʣ����������ƣ�
С�죺���ݽ��ۣ���b����c������Һ��pH��֪��Һ������ԣ��ɷ�̪������Ե���Һ�еı�ɫ������
ȡ����Һ�������еμ�ʯ����Һ������Һ��죬��֪��Һ�����ԣ���֪��������ǹ����ģ��ܰ���������ȫ����Ӧ�������һ���ʣ�࣬������Һ�е����ʷ�����������ӣ�
��2������Ũ����������Լ�Ũ����ϡ����ע�����������գ�
��3���ٸ������ߵ�������7�������ж�����ҺΪ������Һ�����ݷ�Ӧԭ��д���÷�Ӧ�Ļ�ѧ����ʽ��
��С�����������֪����ɫ������������ͭ���ǿ����Ե�ͭ�����������Ʒ�Ӧ���ɵģ��ɴ˿����Ƴ�ԭ��Һ����ʣ����������ƣ�
С�죺���ݽ��ۣ���b����c������Һ��pH��֪��Һ������ԣ��ɷ�̪������Ե���Һ�еı�ɫ������
ȡ����Һ�������еμ�ʯ����Һ������Һ��죬��֪��Һ�����ԣ���֪��������ǹ����ģ��ܰ���������ȫ����Ӧ�������һ���ʣ�࣬������Һ�е����ʷ�����������ӣ�
����⣺��1��ͼ��a��b�������������Ʒֱ��Dz���������Ͳ���������������Ͳ��
��2��ϡ��Ũ����ʱ��Ҫ��Ũ�������������ڻ���ע��ˮ�У����ò��������Ͻ��裻���Ũ���
��3�������ߵ�������7��˵��Ϊ������Һ�����Կ����жϸ�С����������������Һ�еμ�ϡ���ᣬ��M���������ƣ��÷�Ӧ�Ļ�ѧ����ʽΪ��2NaOH+H2SO4�TNa2SO4+2H2O���������������Һ��2NaOH+H2SO4�TNa2SO4+2H2O��
������������Һ��ϡ���ᷢ���кͷ�Ӧ�����Ȼ��ƺ�ˮ��ǡ����ȫ��Ӧʱ��pH����7���������ƹ���ʱ�Լ��ԣ�ϡ�������ʱ�����ԣ��������֪����Ӧ�����Һ����һ����Һ��������ɫ��������ɫ������������ͭ���������Һ�ǿ����Ե�ͭ�Σ����磺CuSO4��CuCl2��Cu��NO3��2�ȣ������Ƴ�ԭ��Һ��ʣ����������ƣ���ҺӦ��a����Һ��
���ݽ��ۣ�b����c������Һ����Һ��pH����7��С��7����Һ�����Ժ����ԣ���������ɫ��̪��Һʱ����Һ����ɫ��
ȡ����Һ�������еμ�ʯ����Һ������Һ��죬��֪��Һ�����ԣ���֪��������ǹ����ģ��ܰ���������ȫ����Ӧ�������һ���ʣ�࣬��Һ�е������������ƺ����ᣬ�����������Na+��H+��SO42-��
�ʴ�Ϊ��CuSO4����Һ����ɫ��ȡ��������Һ�������еμ�ʯ����Һ����Һ��죻Na+��H+��SO42-��
��2��ϡ��Ũ����ʱ��Ҫ��Ũ�������������ڻ���ע��ˮ�У����ò��������Ͻ��裻���Ũ���
��3�������ߵ�������7��˵��Ϊ������Һ�����Կ����жϸ�С����������������Һ�еμ�ϡ���ᣬ��M���������ƣ��÷�Ӧ�Ļ�ѧ����ʽΪ��2NaOH+H2SO4�TNa2SO4+2H2O���������������Һ��2NaOH+H2SO4�TNa2SO4+2H2O��
������������Һ��ϡ���ᷢ���кͷ�Ӧ�����Ȼ��ƺ�ˮ��ǡ����ȫ��Ӧʱ��pH����7���������ƹ���ʱ�Լ��ԣ�ϡ�������ʱ�����ԣ��������֪����Ӧ�����Һ����һ����Һ��������ɫ��������ɫ������������ͭ���������Һ�ǿ����Ե�ͭ�Σ����磺CuSO4��CuCl2��Cu��NO3��2�ȣ������Ƴ�ԭ��Һ��ʣ����������ƣ���ҺӦ��a����Һ��
���ݽ��ۣ�b����c������Һ����Һ��pH����7��С��7����Һ�����Ժ����ԣ���������ɫ��̪��Һʱ����Һ����ɫ��
ȡ����Һ�������еμ�ʯ����Һ������Һ��죬��֪��Һ�����ԣ���֪��������ǹ����ģ��ܰ���������ȫ����Ӧ�������һ���ʣ�࣬��Һ�е������������ƺ����ᣬ�����������Na+��H+��SO42-��
�ʴ�Ϊ��CuSO4����Һ����ɫ��ȡ��������Һ�������еμ�ʯ����Һ����Һ��죻Na+��H+��SO42-��
������Ũ������ˮ��ų��������ȣ�����ΪŨ������ܶȱ�ˮ�����ϡ��Ũ����ʱӦ�ð�Ũ����������ע�뵽ˮ�У����ò��������ϵĽ��裬ʹ������������ʱ��ɢ������

��ϰ��ϵ�д�
�����Ŀ
 ����Ĵ��������Ҳ���������������̽����һЩ���ﴢ������������Ŀǰ��Щ�����������ٶȣ����˹���һЩ����Ŀ���������ͼ��ʾ�������������̽�����ﴢ����һЩ���ҽ����������ͽ����Ļ������õȣ��ش����⣺
����Ĵ��������Ҳ���������������̽����һЩ���ﴢ������������Ŀǰ��Щ�����������ٶȣ����˹���һЩ����Ŀ���������ͼ��ʾ�������������̽�����ﴢ����һЩ���ҽ����������ͽ����Ļ������õȣ��ش����⣺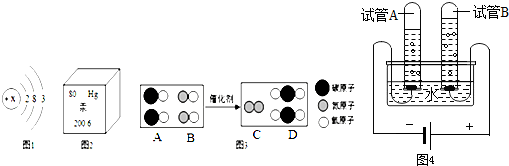
 ��������������������������أ�
��������������������������أ�