��Ŀ����
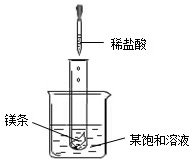
NaNO2���������ƣ���NaCl������M �������ᾧˮ�����ܽ��������ͼ1��ʾ�������ͼ����й���Ϣ�ش��������⣺
��1��t2��ʱ��NaNO2��NaCl��M���ܽ���ɴ�С��˳����______��
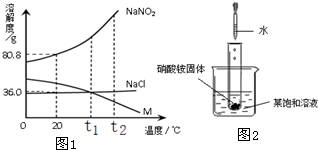
��2����ͼ2��ʾ�������Թ��м���5mLϡ����ʱ���Թ������̲����������ݣ�ͬʱ����ʹ�ձ��б�����Һ����ǣ�������ˮ�������������жϣ��ñ�����Һ�е�������______�����NaNO2����NaCl����M����
��3���ֱ�NaNO2��NaCl��M�ı�����Һ��t2�潵�µ�t1��ʱ��������Һ�����ʵ����������ɴ�С��˳����______��
��4��NaNO2�ж�������ۺ���ζ��ʳ�κ����ƣ����Ҫ��ֹ����ʳNaNO2�������ж��¹ʣ�Ϊ������NaNO2��NaCl���壬ij��ѧ��ȤС��ͬѧ�������ϻ��������Ϣ��NaNO2��ˮ��Һ�ʼ��ԣ�NaCl��ˮ��Һ�����ԣ�NaNO2�۵�Ϊ271�棬NaCl�۵�Ϊ801�森
��С��ͬѧ��Ƶļ���ʵ�鷽�����£�
20��ʱ�ֱ�ȡNaNO2��NaCl��5.0g����ֻС�ձ��У��ֱ����10mLˮ��ˮ���ܶȽ��ƿ���1g/cm3�����ò�������ֽ����۲죮����Ϊ�˷����Ƿ����______������С������С�����
�ڸ�С������ͬѧ����Ƴ���С����ͬ��ʵ�鷽��������óɹ�������Ϊ�÷���������______��ֻ��дһ�ַ���������������

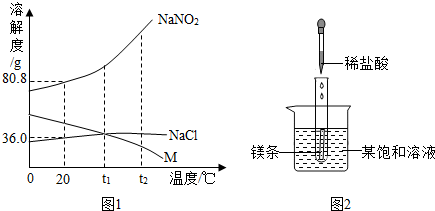
��1��t2��ʱ��NaNO2��NaCl��M���ܽ���ɴ�С��˳����______��
��2����ͼ2��ʾ�������Թ��м���5mLϡ����ʱ���Թ������̲����������ݣ�ͬʱ����ʹ�ձ��б�����Һ����ǣ�������ˮ�������������жϣ��ñ�����Һ�е�������______�����NaNO2����NaCl����M����
��3���ֱ�NaNO2��NaCl��M�ı�����Һ��t2�潵�µ�t1��ʱ��������Һ�����ʵ����������ɴ�С��˳����______��
��4��NaNO2�ж�������ۺ���ζ��ʳ�κ����ƣ����Ҫ��ֹ����ʳNaNO2�������ж��¹ʣ�Ϊ������NaNO2��NaCl���壬ij��ѧ��ȤС��ͬѧ�������ϻ��������Ϣ��NaNO2��ˮ��Һ�ʼ��ԣ�NaCl��ˮ��Һ�����ԣ�NaNO2�۵�Ϊ271�棬NaCl�۵�Ϊ801�森
��С��ͬѧ��Ƶļ���ʵ�鷽�����£�
20��ʱ�ֱ�ȡNaNO2��NaCl��5.0g����ֻС�ձ��У��ֱ����10mLˮ��ˮ���ܶȽ��ƿ���1g/cm3�����ò�������ֽ����۲죮����Ϊ�˷����Ƿ����______������С������С�����
�ڸ�С������ͬѧ����Ƴ���С����ͬ��ʵ�鷽��������óɹ�������Ϊ�÷���������______��ֻ��дһ�ַ���������������
��1���������ʵ��ܽ�����߿�֪����t2��ʱ�������ʵ��ܽ�ȴ�С��ϵ��NaNO2��NaCl��M��
��2��þ��ϡ���ᷴӦ�ų��������ȣ��������֪������ʱ�����ʵı�����Һ�г��ֻ��ǣ�˵�������ʵ��ܽ�����¶ȵ����߶���С���ʸñ�����Һ�е�������M��
��3����t2��ʱ�������ʵ��ܽ�ȴ�С��ϵΪ��NaNO2��NaCl��M����������ʵı�����Һ�����ʵ�����������С��ϵΪ��NaNO2��NaCl��M����������t1��ʱ������NaNO2��NaCl���ܽ�����¶ȵĽ��Ͷ���С��������ǵı�����Һ�оͻ��о�������������Һ�Ծ��DZ�����Һ��������t1��ʱ��NaNO2��NaCl���ܽ�ȴ���˽����Ժ����Һ�����ʵ�����������С��ϵ��NaNO2��NaCl��������M���ܽ�����¶ȵĽ��Ͷ�������˽���ʱ��M�ı�����Һ�ͻ��ɲ�������Һ�������ʵ������������䣬��Ȼ��t1��ʱ��NaCl��M���ܽ����ȣ�������M���Dz�������Һ�������Һ�����ʵ�����������С��ϵ��NaCl��M��
��4��20��ʱ��NaNO2��NaCl���ܽ�ȷֱ�Ϊ80.8g��36g������10mlˮ�м���5g�������ʣ���ȫ���ܽ�NaNO2������ȫ���ܽ����Ȼ��ƣ��ʷ������У�������ѡ�÷ֱ�ȡ������NaNO2��NaCl��������֧�Թ��У��þƾ��Ƽ��ȣ��ۻ���ΪNaNO2��������ΪNaCl����ֱ�ȡ������Ʒ���Թ��У���ˮ�ܽ⣬�μ���ɫ��̪��Һ�������ΪNaNO2��������ΪNaCl����
�ʴ�Ϊ��
��1��NaNO2��NaCl��M
��2��M
��3��NaNO2��NaCl��M
��4���ٿ��� �ڷֱ�ȡ������Ʒ���Թ��У���ˮ�ܽ⣬�μ���ɫ��̪��Һ�������ΪNaNO2��������ΪNaCl���ֱ�ȡ������NaNO2��NaCl��������֧�Թ��У��þƾ��Ƽ��ȣ��ۻ���ΪNaNO2��������ΪNaCl���������������𰸣�

��ϰ��ϵ�д�
�����Ŀ
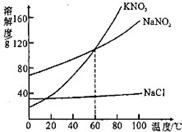 ��1��KNO3��NaNO2��NaCl�������ʵ��ܽ�����¶ȵ�Ӱ��������
��1��KNO3��NaNO2��NaCl�������ʵ��ܽ�����¶ȵ�Ӱ��������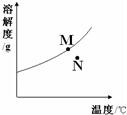 ת�����ǣ�A����Һ������ʱ�����ᾧˮ�� ��
ת�����ǣ�A����Һ������ʱ�����ᾧˮ�� ��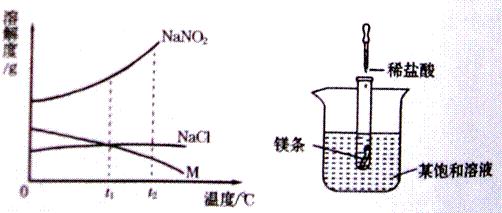
 ��2009?���Ƹۣ� NaNO2���������ƣ���NaCl������M �������ᾧˮ�����ܽ��������ͼ��ʾ�������ͼ����й���Ϣ�ش��������⣺
��2009?���Ƹۣ� NaNO2���������ƣ���NaCl������M �������ᾧˮ�����ܽ��������ͼ��ʾ�������ͼ����й���Ϣ�ش��������⣺