��Ŀ����
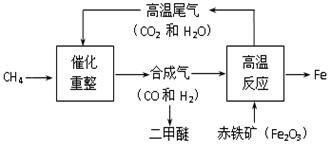
�������������������й㷺����;�������Ϻ���������й��ݡ���������֮�ڡ��ĺ���Ͳ�������ĸ����ֽ�������Ƴɵģ��������˴����ĸ�����

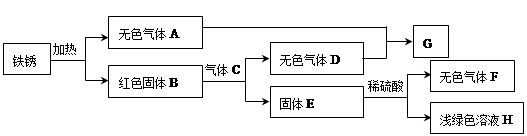
��1���������� ����������������
��2�����������Խ�̿����������Ҫ�ɷ���������Fe2O3����������Ϊ��Ҫԭ����������д��һ����̼���������ڸ����·�Ӧ�Ļ�ѧ����ʽ�� ��
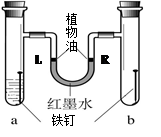
��3��ÿ�������ϸ����IJ����ܸߣ���������ʴҲ����������˾����ʧ�����ڿ�������ʴ��ʵ���������������е� �� ��ͬ���õĽ����
��4��Ϊ�˷�ֹ������ʴ�����dz����� �ķ�������дһ�֣����ﵽĿ�ġ�
(5) �г�����һ�ֺ����۵���Ƭ��ʳ�ú���θ�������½�����ת��Ϊ��������յ���Ԫ�أ���Ӧ�Ļ�ѧ����ʽΪ ��������Ƭ������ձ��棬ԭ����
��

��1���������� ����������������
��2�����������Խ�̿����������Ҫ�ɷ���������Fe2O3����������Ϊ��Ҫԭ����������д��һ����̼���������ڸ����·�Ӧ�Ļ�ѧ����ʽ�� ��
��3��ÿ�������ϸ����IJ����ܸߣ���������ʴҲ����������˾����ʧ�����ڿ�������ʴ��ʵ���������������е� �� ��ͬ���õĽ����
��4��Ϊ�˷�ֹ������ʴ�����dz����� �ķ�������дһ�֣����ﵽĿ�ġ�
(5) �г�����һ�ֺ����۵���Ƭ��ʳ�ú���θ�������½�����ת��Ϊ��������յ���Ԫ�أ���Ӧ�Ļ�ѧ����ʽΪ ��������Ƭ������ձ��棬ԭ����
��
(1) ����� (2) 3CO+ Fe2O3 2 Fe+3CO2
Fe+3CO2
(3) ������O2����ˮ�� H2O�� �� ˮ��H2O����������O2�� (4) ���ᣨ�����𰸾��ɣ�(5) Fe+2HCl=FeCl2+H2����ֹ����������ˮ�����ȷ�Ӧ������
 Fe+3CO2
Fe+3CO2 (3) ������O2����ˮ�� H2O�� �� ˮ��H2O����������O2�� (4) ���ᣨ�����𰸾��ɣ�(5) Fe+2HCl=FeCl2+H2����ֹ����������ˮ�����ȷ�Ӧ������
��1�����к�̼���ϸ�ʱ��������������̼���ϵ�ʱ�����ڸ֣����Ը������ڻ���
��2��һ����̼���������ڸ����·�Ӧ�Ļ�ѧ����ʽΪ��3CO+Fe2O3 2Fe+3CO2��
2Fe+3CO2��
��3�����ڿ�������ʴ��ʵ���������������е�������ˮ��ͬ���õĽ����
��4���ڸ�������Ϳ�ᡢ��һ������ȷ������Է�ֹ�������⣻
��5��θ�����Ҫ�ɷ�����������Ӧ������������ѧ��Ӧʽ�ǣ�2HCl+Fe=H2��+FeCl2������������е�����ˮ��Ӧʹ�����ʣ�
��2��һ����̼���������ڸ����·�Ӧ�Ļ�ѧ����ʽΪ��3CO+Fe2O3
 2Fe+3CO2��
2Fe+3CO2����3�����ڿ�������ʴ��ʵ���������������е�������ˮ��ͬ���õĽ����
��4���ڸ�������Ϳ�ᡢ��һ������ȷ������Է�ֹ�������⣻
��5��θ�����Ҫ�ɷ�����������Ӧ������������ѧ��Ӧʽ�ǣ�2HCl+Fe=H2��+FeCl2������������е�����ˮ��Ӧʹ�����ʣ�

��ϰ��ϵ�д�
�����Ŀ