��Ŀ����
�������������������Ӧ��ʮ�ֹ㷺��
��1����ͼ�ǽ���ͭ��һ��Ӧ��ʵ���������˽���ͭ��_______�ԡ�

��2������Ʒ�ڿ����в�����ʴ��ԭ�������С����ұ��������ã��������ܵı���Ĥ�����ڿ����С����ұ�������Ӧ�Ļ�ѧ����ʽΪ______________��
��3������Ʒ��������ʴ��
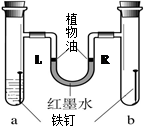
��ijͬѧ�������ͼ��ʾ��װ��̽������ʴ��������a����װ��������е�����ˮ��b�����Ǹ���Ŀ���������L����R�˵�Һ��߶�һ�£�����ľ����һ��ʱ���۲졣�����ƶϴ������ ��
��Ϊ�˷�ֹ����Ʒ���⣬���˱��������������������γɱ���Ĥ�⣬������ ��
��4����ֻ��һ�ֽ������ʣ���������Һ������֤п��ͭ�������ֽ������˳������֤���������漰�Ļ�ѧ����ʽΪ____________________________������˿������ͭ��Һ��Ӧ��ɡ�ͭ����ʵ��ʱ����Ҫ��ȥ��˿���������Ĥ�����õ�����������__________������ϡ���ᴦ����������_________����ʱ����������Ĥ�ѱ��ƻ���
��5���ϳ�����CO��H2���������Ʊ�����Ϊ21��������ȼ�ϵĶ����ѣ�CH3OCH3������������ұ���������䲿����������ʾ��ͼ���£�
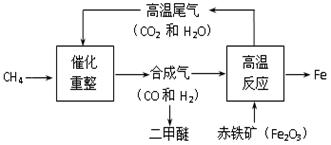
�ٶ����ѣ�CH3OCH3�����ɺϳ�����CO��H2����һ�����������Ƶá���Ӧ������CO��H2�����Ӹ�����1��2���з�Ӧ���Ƶö�����ʱ�����ɵ���һ�������� ��
�ڸ���ʾ��ͼ�б�ʾ�����ʣ��ϳ�����ұ���������������з�����������Ҫ�ķ�Ӧ�����в������û���Ӧ�Ļ�ѧ����ʽΪ ��
��1����ͼ�ǽ���ͭ��һ��Ӧ��ʵ���������˽���ͭ��_______�ԡ�

��2������Ʒ�ڿ����в�����ʴ��ԭ�������С����ұ��������ã��������ܵı���Ĥ�����ڿ����С����ұ�������Ӧ�Ļ�ѧ����ʽΪ______________��
��3������Ʒ��������ʴ��
��ijͬѧ�������ͼ��ʾ��װ��̽������ʴ��������a����װ��������е�����ˮ��b�����Ǹ���Ŀ���������L����R�˵�Һ��߶�һ�£�����ľ����һ��ʱ���۲졣�����ƶϴ������ ��

| A��ֻ��a���е���������ʴ |
| B��L����R�˵�Һ��߶���Ȼ����һ�� |
| C����ʵ�鲻��˵��������������й� |
| D����ʵ��˵��������ʴ�������ˮ�й� |
��4����ֻ��һ�ֽ������ʣ���������Һ������֤п��ͭ�������ֽ������˳������֤���������漰�Ļ�ѧ����ʽΪ____________________________������˿������ͭ��Һ��Ӧ��ɡ�ͭ����ʵ��ʱ����Ҫ��ȥ��˿���������Ĥ�����õ�����������__________������ϡ���ᴦ����������_________����ʱ����������Ĥ�ѱ��ƻ���
��5���ϳ�����CO��H2���������Ʊ�����Ϊ21��������ȼ�ϵĶ����ѣ�CH3OCH3������������ұ���������䲿����������ʾ��ͼ���£�

�ٶ����ѣ�CH3OCH3�����ɺϳ�����CO��H2����һ�����������Ƶá���Ӧ������CO��H2�����Ӹ�����1��2���з�Ӧ���Ƶö�����ʱ�����ɵ���һ�������� ��
�ڸ���ʾ��ͼ�б�ʾ�����ʣ��ϳ�����ұ���������������з�����������Ҫ�ķ�Ӧ�����в������û���Ӧ�Ļ�ѧ����ʽΪ ��
��1�������� ��2��4Al+3O2====2Al2O3 ��3���� B C ���Ƴɲ���֣����ƳɺϽ� ��4�� Cu+2Ag(NO3)3=====Cu(NO3)2+2Ag ��ɰֽ��ĥ
�����ݲ��� ��5����H2O ��3CO+Fe2O3 ���� 2Fe+3CO2
�����ݲ��� ��5����H2O ��3CO+Fe2O3 ���� 2Fe+3CO2
��1������ͭ�������ߣ���Ӧ����ͭ���е����ԣ�
��2�����ڳ����¼��������������������Ӧ����������γ�һ�����ܵı���Ĥ����Ӧ�Ļ�ѧ����ʽΪ4Al+3O2�T2Al2O3��
��3����ͼʾװ����a����װ��������е�����ˮ����a�������ȽӴ�ˮ�ֽӴ�������b�����Ǹ���Ŀ�������b��������ֻ�������Ӵ���������ʴ������a��������������ʴ��ʹ����������٣�b��������û�����Ա仯���������岻�䣬��˿ɹ۲쵽��ֻ��a���е���������ʴ��L��Һ��߶ȸ���R�˵ĸ߶ȣ�ʵ��˵����������ʴ�������ˮ�йأ���BC���ƶ��Ǵ���ģ�
�ڲ�ȡ�ڽ��������γɱ������ı�����ڲ��ṹ�Ƴɲ���ֵķ������ɷ�ֹ������ʴ��
��4����ȡ����ȡ�С��ķ�����ֻ��һ�ֽ������ʼ�����ͭ����������Һ����������������п��Һ����֤п��ͭ�������ֽ������˳����֤������ͭ����������Ӧ��������ͭ��������ѧ����ʽΪCu+2Ag��NO3��3�TCu��NO3��2+2Ag��
Ϊ��ȥ�����������������Ĥ���ɲ�ȡɳֽ��ĥ�ķ�����Ҳ����������������ϡ���ᷴӦ�����������г�ȥ����������ȫ����ȥʱ���ɹ۲��������ᷴӦ�ų��������������ݣ�
��5���ٷ�Ӧ������CO��H2�����Ӹ�����1��2���з�Ӧ������ӦǰC��H��Oԭ�Ӹ�����=1��4��1����������֮һ�Ķ����ѣ�CH3OCH3����C��H��Oԭ�Ӹ�����=2��6��1�����жϷ�Ӧ���ɵ���һ��������H��O������Ϊ2��1�����������Ļ�ѧʽΪH2O��
�ںϳ�����ұ���������������з�����������Ӧ��һΪ������ԭ���������÷�ӦΪ�û���Ӧ��һΪһ����̼��ԭ���������÷�Ӧ�������û���Ӧ����Ӧ���ɶ�����̼��������Ӧ�Ļ�ѧ����ʽΪ
3CO+Fe2O3 ���� 2Fe+3CO2��
��2�����ڳ����¼��������������������Ӧ����������γ�һ�����ܵı���Ĥ����Ӧ�Ļ�ѧ����ʽΪ4Al+3O2�T2Al2O3��
��3����ͼʾװ����a����װ��������е�����ˮ����a�������ȽӴ�ˮ�ֽӴ�������b�����Ǹ���Ŀ�������b��������ֻ�������Ӵ���������ʴ������a��������������ʴ��ʹ����������٣�b��������û�����Ա仯���������岻�䣬��˿ɹ۲쵽��ֻ��a���е���������ʴ��L��Һ��߶ȸ���R�˵ĸ߶ȣ�ʵ��˵����������ʴ�������ˮ�йأ���BC���ƶ��Ǵ���ģ�
�ڲ�ȡ�ڽ��������γɱ������ı�����ڲ��ṹ�Ƴɲ���ֵķ������ɷ�ֹ������ʴ��
��4����ȡ����ȡ�С��ķ�����ֻ��һ�ֽ������ʼ�����ͭ����������Һ����������������п��Һ����֤п��ͭ�������ֽ������˳����֤������ͭ����������Ӧ��������ͭ��������ѧ����ʽΪCu+2Ag��NO3��3�TCu��NO3��2+2Ag��
Ϊ��ȥ�����������������Ĥ���ɲ�ȡɳֽ��ĥ�ķ�����Ҳ����������������ϡ���ᷴӦ�����������г�ȥ����������ȫ����ȥʱ���ɹ۲��������ᷴӦ�ų��������������ݣ�
��5���ٷ�Ӧ������CO��H2�����Ӹ�����1��2���з�Ӧ������ӦǰC��H��Oԭ�Ӹ�����=1��4��1����������֮һ�Ķ����ѣ�CH3OCH3����C��H��Oԭ�Ӹ�����=2��6��1�����жϷ�Ӧ���ɵ���һ��������H��O������Ϊ2��1�����������Ļ�ѧʽΪH2O��
�ںϳ�����ұ���������������з�����������Ӧ��һΪ������ԭ���������÷�ӦΪ�û���Ӧ��һΪһ����̼��ԭ���������÷�Ӧ�������û���Ӧ����Ӧ���ɶ�����̼��������Ӧ�Ļ�ѧ����ʽΪ
3CO+Fe2O3 ���� 2Fe+3CO2��

��ϰ��ϵ�д�
�����Ŀ