��Ŀ����
��ҵ�����г�����������NaCl���ʡ���ͼ�Dzⶨ������Ʒ��Na2CO3����������ʵ��װ�ã����������ã���Ʒ����Ϊ11.0 g��װ��D������Ϊ172.2 g������������Ϊ������Ʒװ����ƿ�С���ֹˮ�У�����������������ӡ�����װ��D���ر�ֹˮ�У���ʢ����Ʒ�Ĺ��ƿ�еμ�ϡ���������ٲ������ݡ���ֹˮ�У��ٻ���������������ӡ�����װ��D������Ϊ176.6 g��������ÿ��װ�þ���Ӧ��ȫ����
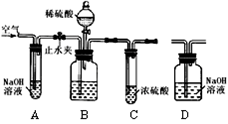
����ȥ��Cװ�ã���ⶨ��� ������ţ���
��ƫ�� ��ƫС �۲��� �����ж�
�ƿ�ʼʱ�ͽ���ʱ��Ҫ����������������ӣ���Ŀ�ķֱ���
��
�Ǽ��㴿����Ʒ��Na2CO3������������

����ȥ��Cװ�ã���ⶨ��� ������ţ���
��ƫ�� ��ƫС �۲��� �����ж�
�ƿ�ʼʱ�ͽ���ʱ��Ҫ����������������ӣ���Ŀ�ķֱ���
��
�Ǽ��㴿����Ʒ��Na2CO3������������
��
�ƿ�ʼʱ���ų�װ���е�CO2�����������CO2�Խ����Ӱ�죻
����ʱ��ʹ���ɵ�CO2ȫ����NaOH��Һ����
�� 96.4%
�ƿ�ʼʱ���ų�װ���е�CO2�����������CO2�Խ����Ӱ�죻
����ʱ��ʹ���ɵ�CO2ȫ����NaOH��Һ����
�� 96.4%
��1��Ũ���������ˮ���������������������̼�ģ��������Ũ���ᣬ�ͻὫ������̼�е�ˮ������Ϊ�Ƕ�����̼���ᵼ�¶�����̼������ƫ���������̼��������Ҳƫ�ʽ��ƫ��
��2����ʼʱ���ų�װ���е�CO2�����������CO2�Խ����Ӱ�죻����ʱ��ʹ���ɵ�CO2ȫ����NaOH��Һ����
��3����ͼ����֪����װ��D�������ն�����̼�ģ�����Dװ�õ������ӵ�������Ϊ������̼�����������Ը��ݶ�����̼��������ϴ�����ϡ���ᷴӦ�Ļ�ѧ����ʽ�����̼���Ƶ��������������̼���Ƶ�����������
�⣺����Ʒ��Na2CO3������Ϊx��
Na2CO3 + H2SO4 Na2SO4 + H2O + CO2��
44
x 176.6 g-172.2 g
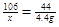 x="10.6" g
x="10.6" g
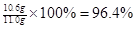
��2����ʼʱ���ų�װ���е�CO2�����������CO2�Խ����Ӱ�죻����ʱ��ʹ���ɵ�CO2ȫ����NaOH��Һ����
��3����ͼ����֪����װ��D�������ն�����̼�ģ�����Dװ�õ������ӵ�������Ϊ������̼�����������Ը��ݶ�����̼��������ϴ�����ϡ���ᷴӦ�Ļ�ѧ����ʽ�����̼���Ƶ��������������̼���Ƶ�����������
�⣺����Ʒ��Na2CO3������Ϊx��
Na2CO3 + H2SO4 Na2SO4 + H2O + CO2��
44
x 176.6 g-172.2 g
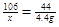 x="10.6" g
x="10.6" g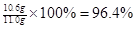

��ϰ��ϵ�д�
�����Ŀ
 Ti + 2MgCl2����Ҫ�Ƶ�12 g�ѣ���Ҫþ�������Ƕ��ٿˣ�
Ti + 2MgCl2����Ҫ�Ƶ�12 g�ѣ���Ҫþ�������Ƕ��ٿˣ�