��Ŀ����
����Ŀ��ˮ��һ����Ҫ����Ȼ��Դ��������������������ʡ���ش��������⣺
��1����Ȼ��ˮһ��������ԣ���ԭ���ǣ�_____���û�ѧ����ʽ��ʾ����ʹ��ָ������Ե���ķ�����_____��
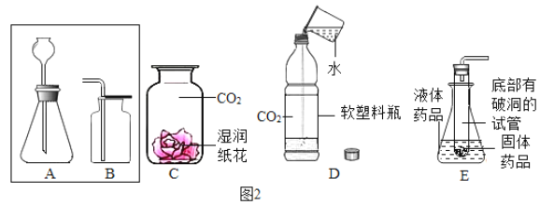
��2����ͼ��ʵ��Ŀ����_____��ʵ���г�����һЩ���ᣬĿ����_____�����Թ�1���ռ���8mL����ʱ���Թ�2���ռ������������Ϊ_____mL��ijͬѧȡ50g��������Ϊ2%��������Һ���е�⣬��������Һ������������Ϊ2.5%ʱ������ˮ������Ϊ_____��
��3������ˮ�ǹ��ʹ��ϵġ��ڶ�ˮԴ�����ǽ�����������ˮ����������������һ����Χ��ʹ�õ�ˮ����������ˮ����Ҫ��������ͼ��
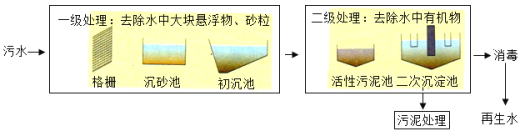
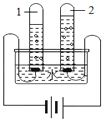
��һ������ʱ����ˮ������դ���������м�������������դ��������_____��������������_____��
�ڶ�������ʱ����Ҫʹ�û���̿������������_____��
�۶��δ������ˮ�����������������õ�����ˮ����������ˮ�Ƿ�ΪӲˮ������ʹ�õ�������_____��
���𰸡�CO2+H2O===H2CO3 ������� ̽��ˮ����� ��ǿˮ�ĵ����� 4.0 10g ���� ���� ����ɫ�غ���ζ ����ˮ
��������
��1����Ȼ��ˮһ��������ԣ�ԭ���Ƕ�����̼�ܽ���ˮ�У�ʹ��ˮ�����ԣ���ѧ����ʽΪ��CO2+H2O===H2CO3��ʹ��ָ������Ե���ķ����Ǽ�����С����CO2+H2O===H2CO3���������
��2������һ�����ˮ��ʵ��װ��ͼ����ʵ��Ŀ����̽��ˮ����ɣ�ʵ���г�����һЩ���ᣬ�������ƣ�Ŀ����Ϊ����ǿ��Һ�ĵ����ԣ����Թ�1���ӵ�Դ�ĸ������ռ��������������ռ���8mL����ʱ���Թ�2���ռ����������������Ϊ���������һ��4mL��ijͬѧȡ50g��������Ϊ2%��������Һ���е�⣬��������Һ������������Ϊ2.5%ʱ�����������Һ������ΪX��![]() ���X=40g��������ˮ������Ϊ50g-40g=10g�������ǿˮ�ĵ����ԣ�4��10g
���X=40g��������ˮ������Ϊ50g-40g=10g�������ǿˮ�ĵ����ԣ�4��10g
��3����һ������ʱ����ˮ������դ���������м���������������������դ�����������˵����ã�������������������������ˣ�����
�ڶ�������ʱ����Ҫʹ�û���̿������̿�������ɶ�յĽṹ��������������������������������ɫ�غ���ζ�������ȥɫ�غ���ζ
�۶��δ������ˮ�����������������õ�����ˮ����������ˮ�Ƿ�ΪӲˮ������ʹ�õ����ʷ���ˮ�����顣�������࣬��ΪӲˮ����ĭ�࣬��Ϊ��ˮ���������ˮ

 ��У����ϵ�д�
��У����ϵ�д�