��Ŀ����
�����зḻ��ʯ��ʯ��Դ��Ϊ�˲ⶨij��ʯ��ʯ��̼��Ƶ�����������ȡ7.5g��Ʒ�����ձ�������ϡ������ǡ�ò��ٲ�������ʱ����34.7g,�ų��������ڳ��������Ϊ1.1L��
��1��������CO2������ܶ�Ϊ2.0g/L����������Ӧ�ų����������Ϊ g��
��2����ʯ��ʯ�е����ʾ�������ˮ�Ҳ������ᷴӦ������ʯ��ʯ��CaCO3�����������ͷ�Ӧ������Һ�����ʵ������������������ս������1λС������
��1��������CO2������ܶ�Ϊ2.0g/L����������Ӧ�ų����������Ϊ g��
��2����ʯ��ʯ�е����ʾ�������ˮ�Ҳ������ᷴӦ������ʯ��ʯ��CaCO3�����������ͷ�Ӧ������Һ�����ʵ������������������ս������1λС������
��1��2.2
��2��ʯ��ʯ��CaCO3����������Ϊ66.7%
��Ӧ������Һ�����ʵ���������Ϊ14.8%
��2��ʯ��ʯ��CaCO3����������Ϊ66.7%
��Ӧ������Һ�����ʵ���������Ϊ14.8%
��1��������̼������m=��V=2.2g��
��2����̼��Ƶ�����Ϊx�����ɵ��Ȼ��Ƶ�����Ϊy��
��CaCO3+2HCl�TCaCl2+H2O+CO2����
100 73 111 44
x y 2.2g
100:x ="111:y" ="44:2.2g" ��
���x=5g��y=5.55g��
����Ʒ��̼��Ƶ���������Ϊ
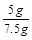 ��100%=66.7%��
��100%=66.7%����3�������ʼȲ�����ˮ��Ҳ�����������ʷ�Ӧ������Һ������Ϊ7.5g+34.7g-2.2g=40g�����ʵ�����Ϊ5.55g�������ʵ���������Ϊ
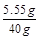 ��100%=14.8%��
��100%=14.8%��
��ϰ��ϵ�д�
�����Ŀ

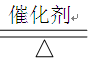 CH4 + 2H2O��������66 g������̼����ת�����õ�����������Ƕ��١�
CH4 + 2H2O��������66 g������̼����ת�����õ�����������Ƕ��١�