��Ŀ����
����Ŀ��Ϊ�˲ⶨij̼������Ʒ�������Ȼ��Ƶ�����������ͬѧ����������ʵ�飬��Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+CaCl2===CaCO3��+2NaCl��ʵ���������±���
��� | ��1�� | ��2�� | ��3�� |
��ȡ������Ʒ������/g | 7 | 5 | 5 |
�����Ȼ�����Һ������/g | 50 | 50 | 75 |
��Ӧ�����ɳ���������/g | 4 | 4 | 4 |
������м��㣺
(1)̼������Ʒ��̼���Ƶ������Ƕ��٣�
(2)������ʵ���г�ַ�Ӧ��������Һ���Ȼ��Ƶ����������Ƕ��٣�
���𰸡���1��4.24�ˣ�2��7.16%
��������
�⣺���ݸ������������ݿ�֪��ֻ�е�2��ʵ��ǡ����ȫ��Ӧ��
��̼���Ƶ�����Ϊx�����ɵ��Ȼ��Ƶ�����Ϊy

x=4.24g
y=4.68g
������ʵ���г�ַ�Ӧ��������Һ���Ȼ��Ƶ���������Ϊ![]()
��
��1��̼������Ʒ��̼���Ƶ�������4.24g��
��2��������ʵ���г�ַ�Ӧ��������Һ���Ȼ��Ƶ���������Ϊ7.16%��

 �����Ļ���������人������ϵ�д�
�����Ļ���������人������ϵ�д� ���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д� ��ٽ������½������������ϵ�д�
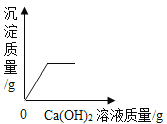
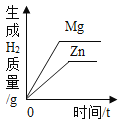
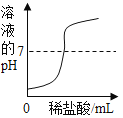
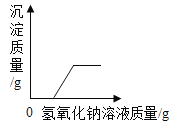
��ٽ������½������������ϵ�д�����Ŀ������ͼ������ȷ��ӳ���Ӧ�������ǣ�������
A | B | C | D |
|
|
|
|
��һ������Na2CO3��Һ����μ���Ca��OH��2��Һ | ��������Zn��Mg�ֱ����������������������ϡ���ᣨ��������Ӧ | ��NaOH��Һ����μ���ϡ���� | ��һ�������������ͭ�����Һ����εμ�����������Һ |
A. AB. BC. CD. D