��Ŀ����
��������ʵ��װ��ͼ���ش����⣺
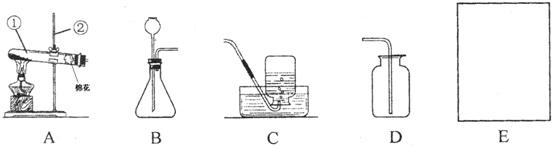
��1��д��ͼ�б���������ƣ���______����______��
��2��ijͬѧ��A��Cװ����ȡ���ռ�һƿ�������Թ�������ҩƷ�Ļ�ѧʽ��______������������______�����۲쵽C�е��ܿڸ�������ð��ʱ�������ռ���������ƿ�г������������ƿ��ˮ����ȡ�������ô����ǵ�ľ������ƿ�У����δ��ľ����ȼ����ԭ�������______��
��3��������Bװ����ȡ��������______��______�ȣ���д����ȡ����һ������Ļ�ѧ����ʽ______����ȡ������ʱ��ƿ�з����������______���ӳ���©���м����������______����Dװ���ռ�������ʱ������Ӧ��______����a����b������ͨ�룮
���𰸡���������1����Ϥ�����������˽����ƣ�
��2����A��Cװ����ȡ���ռ�һƿ������ҩƷ�����Ǹ�����أ����ݸ��������ȡ������ע������ش�
��3��Bװ�������ڹ�-Һ�����ȷ�Ӧ��ȡ�����壻��ƿ�зŹ��壬Һ��ӳ���©�����룮
����⣺��1��ͼ�Т�������̨���ھƾ��ƣ�
�ʴ�Ϊ������̨���ƾ��ƣ�
��2���ܹ�������ȡ������ҩƷ�Ǹ�����أ��仯ѧʽKMnO4�����йܿڷ�һС���������Ƿ�ֹ�ȵ��������Ѹ�����ط�ĩ���뵼�ܣ�δ��ľ����ȼ����ԭ��������ռ��������庬�п����������������㣮
�ʴ�Ϊ��KMnO4�� ��ֹ����ʱ������ط�ĩ���뵼�ܣ��ռ��������庬�п����������������㣻
��3��Bװ�������ڹ�-Һ�����ȷ�Ӧ��ȡ�����壬��CO2��H2��������ȡ������̼�Ļ�ѧ��Ӧʽ�ǣ�CaCO3+2HCl=CaCl2+H2O+CO2������ƿ�з������ʯ��ʯ������©���м���Һ��ϡ����������̼�ܶȴ��ڿ����ܶȣ���Dװ���ռ�ʱ������Ӧ��a�˽���
�ʴ�Ϊ��CO2��H2��CaCO3+2HCl=CaCl2+H2O+CO2����ʯ��ʯ��ϡ���a��
����������ˮ���ռ�����ʱ�����ܿڸ�������ð��ʱ��Ӧ�����ռ�����տ�ʼð�����ǿ�����Ҫ�ȵ��ܿ��о�������������ð��ʱ���ռ���
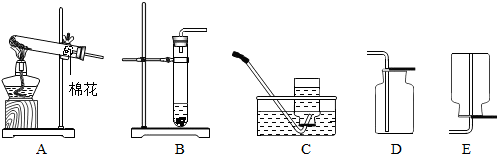
��2����A��Cװ����ȡ���ռ�һƿ������ҩƷ�����Ǹ�����أ����ݸ��������ȡ������ע������ش�
��3��Bװ�������ڹ�-Һ�����ȷ�Ӧ��ȡ�����壻��ƿ�зŹ��壬Һ��ӳ���©�����룮
����⣺��1��ͼ�Т�������̨���ھƾ��ƣ�
�ʴ�Ϊ������̨���ƾ��ƣ�
��2���ܹ�������ȡ������ҩƷ�Ǹ�����أ��仯ѧʽKMnO4�����йܿڷ�һС���������Ƿ�ֹ�ȵ��������Ѹ�����ط�ĩ���뵼�ܣ�δ��ľ����ȼ����ԭ��������ռ��������庬�п����������������㣮
�ʴ�Ϊ��KMnO4�� ��ֹ����ʱ������ط�ĩ���뵼�ܣ��ռ��������庬�п����������������㣻
��3��Bװ�������ڹ�-Һ�����ȷ�Ӧ��ȡ�����壬��CO2��H2��������ȡ������̼�Ļ�ѧ��Ӧʽ�ǣ�CaCO3+2HCl=CaCl2+H2O+CO2������ƿ�з������ʯ��ʯ������©���м���Һ��ϡ����������̼�ܶȴ��ڿ����ܶȣ���Dװ���ռ�ʱ������Ӧ��a�˽���
�ʴ�Ϊ��CO2��H2��CaCO3+2HCl=CaCl2+H2O+CO2����ʯ��ʯ��ϡ���a��
����������ˮ���ռ�����ʱ�����ܿڸ�������ð��ʱ��Ӧ�����ռ�����տ�ʼð�����ǿ�����Ҫ�ȵ��ܿ��о�������������ð��ʱ���ռ���

��ϰ��ϵ�д�
 ����˼ά�żӿ���ϵ�д�
����˼ά�żӿ���ϵ�д� �����Ծ�ϵ�д�
�����Ծ�ϵ�д�
�����Ŀ