��Ŀ����
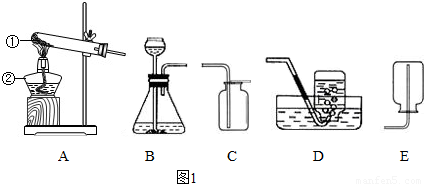
��������ʵ��װ��ͼ1�ش����⣮

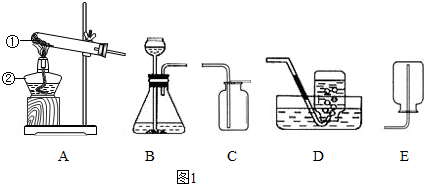
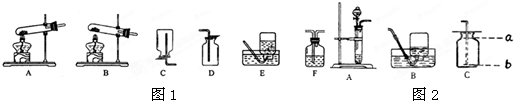
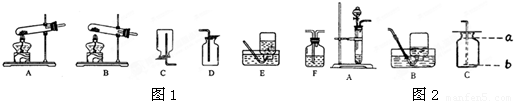
��1������A-Eװ����ȡ������д��������ȡ�����ļ���װ�� �� ������ĸ����
ʵ������ȡ�����Ļ�ѧ����ʽ ��
��2��ʵ������ȡCO2�Ļ�ѧ����ʽΪ ��
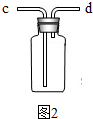
��ͼ2װ����һ���Ʊ�CO2��װ�ã�������װ�������ԵIJ����������ǣ����ü��Ӽ�ס�������е���Ƥ�ܣ�����©���м���ˮ���γ�һ��ˮ�������ã��۲쵽 ��˵�����������ã�
��3����ʪ�����ɫʯ��Сֽ������ʢ��CO2�ļ���ƿ�У��۲쵽�������� ��

��1������A-Eװ����ȡ������д��������ȡ�����ļ���װ��
ʵ������ȡ�����Ļ�ѧ����ʽ
��2��ʵ������ȡCO2�Ļ�ѧ����ʽΪ
��ͼ2װ����һ���Ʊ�CO2��װ�ã�������װ�������ԵIJ����������ǣ����ü��Ӽ�ס�������е���Ƥ�ܣ�����©���м���ˮ���γ�һ��ˮ�������ã��۲쵽
��3����ʪ�����ɫʯ��Сֽ������ʢ��CO2�ļ���ƿ�У��۲쵽��������
����������Ҫ����ʵ������ȡ�����װ�úͷ�Ӧԭ������𣬸��ݷ�Ӧ���״̬�ͷ�Ӧ��������������ȡ����ķ���װ�ã�����������������������ռ�������������װ�õ������Լ��鷽�������
����⣺��1����ȡ������п����ϡ���ᷴӦ����Ҫ���ȣ�����ѡ��Bװ�ã�����������ˮ���ܶȱȿ���С�������ռ������ȿ����������ſ��������ֿ�����ˮ������Ӧԭ����Zn+H2SO4�TZnSO4+H2����
��2��ʵ������ȡCO2�Ļ�ѧ����ʽΪ��CaCO3+2HCl�TCO2��+CaCl2+H2O��
��3��������̼��ˮ����̼�ᣬ̼����ʹʯ���죮
������1��BC��BD��Zn+H2SO4�TZnSO4+H2��
��2��CaCO3+2HCl�TCO2��+CaCl2+H2O��ˮ�����½�
��3����ɫֽ�����
��2��ʵ������ȡCO2�Ļ�ѧ����ʽΪ��CaCO3+2HCl�TCO2��+CaCl2+H2O��
��3��������̼��ˮ����̼�ᣬ̼����ʹʯ���죮
������1��BC��BD��Zn+H2SO4�TZnSO4+H2��
��2��CaCO3+2HCl�TCO2��+CaCl2+H2O��ˮ�����½�
��3����ɫֽ�����
���������⿼������й��������ȡװ�ü���Ӧԭ����Ҫ�����������й��������ȡʵ�鼰ע�����

��ϰ��ϵ�д�
 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д�
�����Ŀ