��Ŀ����
ר����ʾ���ճ���������״�����С�84������Һ����ʹ������
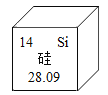
��1������Ч�ɷ�NaClO����Ԫ�صĻ��ϼ�Ϊ____��������������Ϊ____��д���ӷ��ţ���
��2����84������Һ������ԭ��Ϊ2NaClO+CO2+X�TNa2CO3+2HClO�����ɵ�HClO�������ᣩ���н�ǿ��ɱ�����á���ѧ����ʽ��X�Ļ�ѧʽΪ____��
��3����84������Һ��һ���Ĵ̼����븯ʴ�ԣ�����ϡ���Ժ����ʹ�á���50g��NaClO 5%�ġ�84������Һϡ����1%������ԭ��Һ�м���ˮ������Ϊ_________����
��4���ƾ���C2H5OH����������(CH3COOOH)Ҳ��������Ч����¹ڲ����������������߷ֱ�����������ȫȼ�յIJ�������ͬ�ģ���������ɵĽǶȽ�����ԭ����___________��
 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
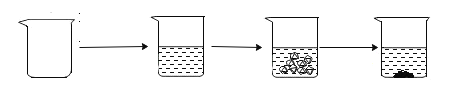
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�ijУ��ѧ��ȤС��Ϊ�ⶨ��ͭ��п��������������������ʵ�飺
ʵ�鲽�� | �ٳ�ȡ�ձ������� | �ڽ�������������ձ��в����� | �۳�ȡ��ͭ��Ʒ�����ձ��У�ʹ֮������ǡ����ȫ��Ӧ | ���ط�Ӧ��ȫ���� |
ʵ��ͼʾ |
| |||
ʵ������ | �ձ�������Ϊ50.0g | �ձ������������Ϊ150.0g | ��ͭ��Ʒ������Ϊ20.0g | �ձ������л�������Ϊ169.6g |
�漰��Ӧ�Ļ�ѧ����ʽ��Zn+2HCl==ZnCl2+H2��������������⣺
��1������ۿɹ۲쵽�������ǣ�_____��
��2����ʵ�������ɵ�������������_____g��
��3����û�ͭ��Ʒ��п������������