��Ŀ����
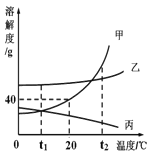
����Ŀ��ij�ο��±ˮ����Ҫ����KCl����������MgSO4��CaCl2�����ʡ��Ӹ�±ˮ����ȡKCl������������ͼ��ʾ��
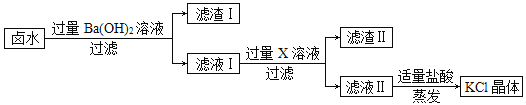
�ش��������⣺
��1��±ˮ��Ba(OH)2��Ӧ�Ļ�ѧ����ʽΪ��____________������____________��Ӧ��
��2��X��Һ�����ʵĻ�ѧʽΪ____________________��
��3�����������Ҫ�ɷ���__________________________��
��4������Һ���м��������Ŀ����____________���ж����������������ǣ�_________�����з����кͷ�Ӧ�Ļ�ѧ����ʽΪ��___________________��
��5�����������У��ò��������Ͻ��裬Ŀ����______________��
���𰸡�MgSO4+Ba(OH)2=BaSO4��+Mg(OH)2�� ���ֽ� K2CO3 BaCO3��CaCO3 ��ȥ��Һ�е��������غ�̼��� �պ�û�����ݲ��� KOH+HCl=KCl+H2O ��ֹ��ֲ��¶ȹ��ߣ����Һ��ɽ�
��������
��1��±ˮ�е�MgSO4��Ba(OH)2��Ӧ����������þ���������ᱵ��������Ӧ�Ļ�ѧ����ʽΪ��MgSO4+Ba(OH)2=BaSO4��+Mg(OH)2�����˷�Ӧ���ڸ��ֽⷴӦ��
��2����ҺI�������������Լ��Ȼ��ƣ���Ȼ��Ҫ�ɷ����Ȼ��أ�����Ҫȥ�������������Ȼ��ƣ���Ҫ����̼��أ�����ȥ�����ʶ��������µĽ������ӣ�����X��Һ�����ʵĻ�ѧʽΪ K2CO3��
��3������ҺI�м���̼�����Һ��̼��غ�ʣ�������������Ӧ����̼�ᱵ�������������أ�̼��غ��Ȼ��Ʒ�Ӧ����̼��Ƴ������Ȼ��ƣ��������������Ҫ�ɷ���BaCO3��CaCO3��
��4�����ڼ����̼��ع�����ͬʱ̼��غ�����������Ӧ�����������أ���������Һ���м�����������Ŀ���dz�ȥ��Һ�е��������غ�̼��أ��ж����������������Ǹպò���������ð������ʱ̼��غ�ϡ����ǡ����ȫ��Ӧ���������غ����ᷴӦ�����Ȼ��غ�ˮ�����кͷ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ��KOH+HCl=KCl+H2O��
��5��������Һ�õ�KCl����Ĺ����У�Ҫ�ò��������Ͻ��裬Ŀ���� ��ֹ��ֲ��¶ȹ��ߣ����Һ��ɽ���

 ��У����ϵ�д�
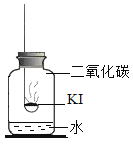
��У����ϵ�д�����Ŀ���⻯�أ�KI�����治������ʡ�ʵ��С���������ʵ��̽��KI���ʵ����ء�
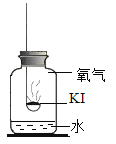
���������1��KI���ʵ�������ʲô��
���������ϣ�
��KIΪ��ɫ��ĩ����¶�ڿ����л���ûᱻ����Ϊ�⣨I2�������Ʊ��ʡ�
�ڵ�ˮ�к��϶�KIʱ���μӵ�����Һ����ɫ������ɫ
������ʵ�飩�ֱ�ȡ����KI��ȼ�ճ��У��ٷֱ����ʢ�в�ͬ���ʵļ���ƿ�У������������������۲졣����ʵ�飺ȡʵ��1�������ƹ����ܽ⣬���������Һ����Һ����ɫ��ȡʵ��4��������ɫ�����ܽ⣬���������Һ����Һ����ɫ��
ʵ��1 | ʵ��2 | ʵ��3 | ʵ��4 |
|
|
|
|
����䳱��������� | �������������� | ����䳱���������������� | ����䳱�������� |
����������ۣ�
��1��ʵ��3��Ŀ����_________________��
��2���Ա�ʵ��__________�����Եó�KI����һ����ˮ�йء�
��3��������ʵ�����֪��KI���ʵ�������_____________��
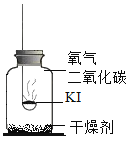
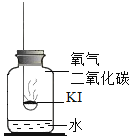
���������2��CO2��������ʲô��
������ʵ�飩�ֱ�ȡ10mLͬŨ�ȵ�KI��Һ��3֧�Թ��У������Թ�2��ͨ��CO2�����Թ�3�еμӼ�������ֱ����Һ��pH�������Ӻ۲���Һ����ɫ�������Թ��е��������Һ���۲���Һ����ɫ��ʵ�������¼���£��ϳ�ʱ��۲쵽�Թ�1����Һ��Ϊ��ɫ��
�Թ���� | 1 | 2 | 3 |
��ҺpH | pH=8.5 | pH=6.5 | pH=4.5 |
��Һ��ɫ | ��ɫ | dz��ɫ | ��ɫ |
�μӵ�����Һ�����ɫ | ��ɫ | ��ɫ | ����ɫ |
����������ۣ�
��4���Թ�1ʵ���Ŀ����____________��
��5��CO2��KI���ʹ����е�������__________________��
����˼�����ۣ�
��6��̽��KI��������ʱ��ͬѧ���ų��˵�����ϡ�������Ӱ�죬��ԭ����_______________��