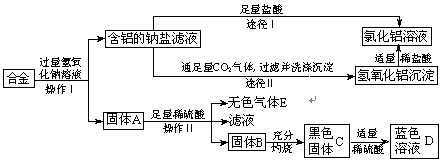
��Ŀ����
ijС������ϡ������ݺ�������ͭ������п�ķ��ϣ�ʵ�ַ�����ۺ����ã���ʵ��������ͼ��ʾ����֪������������ϡ���ᷴӦʱ��Ԫ�ػ��ϼ۲������ı䣬��ͼ�в�����������ȥ����
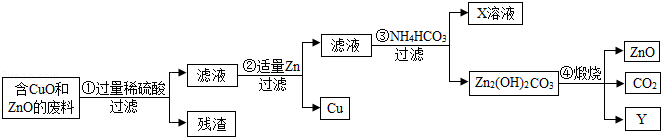
��1���������еĹ��˲�����ʹ�õIJ����������ձ����������� �����в������������� ��
��2����������ͭ��ϡ���ᷴӦ�Ļ�ѧ����ʽ�� ���������ɽ���Cu�Ļ�ѧ����ʽ�� ��
��3��X�Ļ�ѧʽ�� ��
��4������Zn2��OH��2CO3�Ļ�ѧ����ʽ�� ��

��1���������еĹ��˲�����ʹ�õIJ����������ձ����������� �����в������������� ��
��2����������ͭ��ϡ���ᷴӦ�Ļ�ѧ����ʽ�� ���������ɽ���Cu�Ļ�ѧ����ʽ�� ��
��3��X�Ļ�ѧʽ�� ��
��4������Zn2��OH��2CO3�Ļ�ѧ����ʽ�� ��
��1��©������������2��CuO+H2SO4=CuSO4+H2O��Zn+CuSO4=ZnSO4+Cu��
��3����NH4��2SO4����4��Zn2��OH��2CO3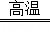 2ZnO+CO2��+H2O��
2ZnO+CO2��+H2O��
��3����NH4��2SO4����4��Zn2��OH��2CO3
 2ZnO+CO2��+H2O��
2ZnO+CO2��+H2O�������������1�����˲�����ʹ�õIJ����������ձ�������������©������������������������
��2������ͭ�����ᷴӦ����������ͭ��ˮ����Ӧ�Ļ�ѧ����ʽ��CuO+H2S04�TCuS04+H20������п�Ļ����Դ���ͭ�����Խ�п������ͭ��Ӧ��ʵ�֡�Zn��Cu��ת�䣬��Ӧ����п������ͭ����������ͭ������п�����Է�Ӧ�Ļ�ѧ����ʽ�ǣ�Zn+CuSO4�TCu+ZnS04��
��2���ɷ�����ۺ���������ͼ�����ʼ�ķ�Ӧ��֪��Һ�к�����������ӣ���̼����立�Ӧ����������泥���������X������泥���ѧʽ�ǣ���NH4��2S04��
��3����Ԫ���غ��֪�����ռ�ʽ̼��п��������п��������̼��ˮ���ù۲취��ƽ����Ӧ�����Ǹ��£����Է���ʽ�ǣ�Zn2��OH��2CO3
 2ZnO+CO2��+H2O
2ZnO+CO2��+H2O
��ϰ��ϵ�д�
�����Ŀ