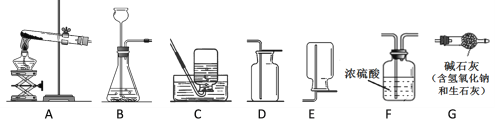
��Ŀ����
����Ŀ��ij�о���ѧϰС��Ϊ�˲ⶨ��ͭ��ͭ��п�Ͻ𣩵���ɡ��ڻ�ѧ��ʦ�İ����£�ѡ��98%��Ũ���ᡢ��ͭ��Ʒ��������ʵ��ͼ��㡣
ʵ�������150g 9.8%��ϡ���ᡣ
��1�����㣺��ҪŨ���������____________g��Լ8.2mL��
��2����ȡ������Ͳȷ��ȡ�����Ũ�����ˮ��
��3�����ȣ�ϡ��ʱһ��Ҫ��____________����������ע��____________������Ͻ��衣
��4��װƿ����ǩ������д�²�ı�ǩ��____________

ʵ��ⶨ��ͭ��Ʒ��ͭ��������������ȡ��ͭ��Ʒ10g����������μ���9.8����
ϡ�������պò��ٲ�������Ϊֹ����Ӧ���������ɵ�����������������Һ��������ϵ��ͼ��ʾ��

�Լ��㣺
��Ʒ��ͭ����������������������ȷ��С�����һλ��______���ڴ����д��������̣�
���𰸡� 15 Ũ���� ˮ ������Һ 9.8% �⣺���ͭ��Ʒ��п������ΪX��
Zn + H2SO4 = ZnSO4 + H2��
65 2
X 0.1g
65��2= X: 0.1g
X=3.25g
��ͭ��Ʒ��ͭ����������Ϊ��10g-3.25g��/10g ��100%=67.5%
�𣺻�ͭ��Ʒ��ͭ����������Ϊ67.5%��
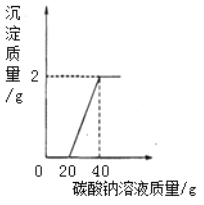
��������ʵ���1�����ݸ�����Һ����ǰ����������������
��3������Ũ����ϡ�͵IJ������裨����ˮ���ر��ڡ������������Ͻ���������
��4�����ݱ�ǩ����дҪ�������
ʵ�������ط�Ӧ�������
�⣺ʵ���1������ҪŨ���������Ϊx��98%x=150g��9.8% x=15g��
��3�����ȣ�ϡ��ʱһ��Ҫ��Ũ��������������ע��ˮ������Ͻ��裻
��4��װƿ����ǩ����ǩ���ݰ������ơ�������������������������Һ 9.8%��
ʵ�����ͼʾ��֪��������������Ϊ0.1g�������������������п�������������ͭ������������������������
���ͭ��Ʒ��п������ΪX��
Zn + H2SO4 = ZnSO4 + H2��
65 2
x 0.1g
![]()
x=3.25g
��ͭ��Ʒ��ͭ����������Ϊ![]() =67.5%
=67.5%
�𣺻�ͭ��Ʒ��ͭ����������Ϊ67.5%��

 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�����Ŀ������ͭ��һ��Ӧ�ü���㷺�Ļ���ԭ����������ˮ���������ھƾ���ͭ������ȫ������pH��5���ң���������ȫ������pH��2����.������ij�����ú�����ͭΪԭ������������CuSO4��5H2O��������ʯ�ࣨCaSO4��2H2O������������ʾ��ͼ��
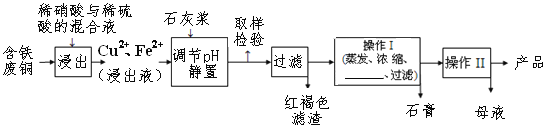
������ʯ���ڲ�ͬ�¶��µ��ܽ�ȣ�g/100gˮ�������
�¶�(��) | 20 | 40 | 60 | 80 | 100 |
ʯ�� | 0.32 | 0.26 | 0.15 | 0.11 | 0.07 |
���� | 32 | 44.6 | 61.8 | 83.8 | 114 |
��ش��������⣺
��1�����ɫ��������Ҫ�ɷ���____________��
��2��ʯ�ҽ���pH��ԼҪ���ڵ�__________
A. 2 B. 5 C. 7 D.10
��3�������ķ�ͭ��ϡ���ᷴӦ�Ļ�ѧ����ʽ________________________________��
��4������I����¶�Ӧ�ÿ�����___________�����ң�
��5������Һ�з��������ͭ����IJ�����ӦΪ����Ũ����__________�����ˡ�ϴ�ӡ������������ˮ�Ҵ���ϴ��Һ����������ˮ��ԭ����_________________��
����Ŀ��С��������20��ʱ̽�����������ˮ�����������������ʵ�飺A�ձ�ʢ��20mLˮ

������ϣ�
������ڲ�ͬ�¶��µ��ܽ�������
�¶ȡ� | 10�� | 20�� | 60�� | 100�� |
�ܽ��/g | 20.9 | 31.6 | 110 | 246 |
���������ܼ������䣬�����й����ϻش����⣺
��1��A��B��B��C�ܽ�����л���Ҫ��________________����һ�������ƣ���
��2������ʵ����������ڱ�����Һ���ǣ�����ĸ��__________________��
��3����B��C��D��E�У���Һ����������������С��___________________������ĸ����