��Ŀ����
����Ŀ����ѧ̽����ѧϰ��ѧ��Ҫ����Ч��ѧϰ��ʽ��ij��ѧ��ȤС���ͬѧ�������Ϊר����������¼���̽��ʵ�顣
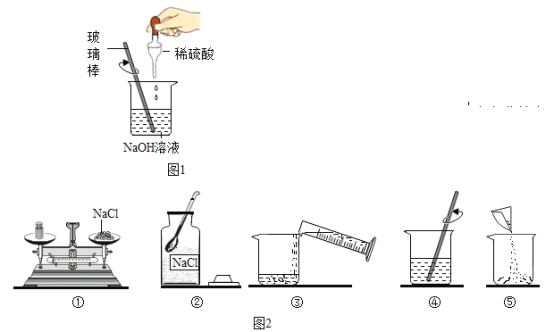
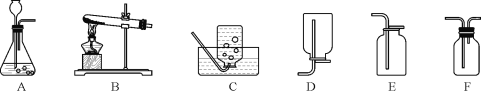
��1����ͼ1��ijͬѧ��Ƶ�̽������Ӧ��ʵ�飬��ش�
��д���÷�Ӧ�Ļ�ѧ����ʽ____________��
�ڸ�ʵ�����û��������������Ӧ�����Һ�еμӷ�̪��Һ����Һ����ɫ��Ҫȷ����Ӧ����Һ�ijɷ֣��ɹ�ѡ�õ��Լ��У�ʯ����Һ������ͭ���塢̼������Һ���Ȼ�����Һ������Ϊʲô�Լ����ܴﵽʵ��Ŀ�ģ�_______
��2���������ý������ᷴӦ��ʵ���У�ijͬѧ������������Һ������μӵ��������У��������Ҳ�����ݲ������������Ͽ�֪������������������Һ��Ӧ����������ƫ�����ƣ� NaAlO2�����û�ѧ����ʽΪ____________��
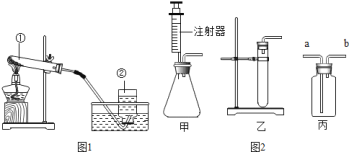
��3��ij��ѧС��ͨ����������Һ�н��е�ʵ�飬̽�����ֽⷴӦ������������
a��HCl��Ca��OH��2
b��H2SO4��K2CO3
c��NaOH��CuSO4
d��Ba��NO3��2��Na2SO4
e��Na2CO3��KOH
������ʵ���У����ܷ������ֽⷴӦ����___��
��ʵ��c�пɹ۲쵽��������________��
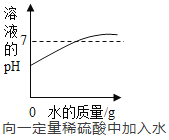
��4����ͼ2��ijͬѧ����150g������������Ϊ7%���Ȼ�����Һ�IJ������̡�
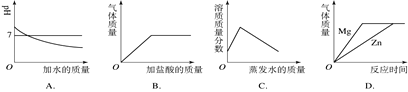
������������һ�����ô���ᵼ����������Һ��������������___7%������������������=����������������ȷ��˳��Ϊ_______��
��5��Ϊ�ⶨ���ֱ��ʵ��ռ����������Ƶ�������������ȡ�ù�����Ʒ10g������50gϡ�����У�ǡ����ȫ��Ӧ����Ӧ����Һ������Ϊ57.8g�����㣺����Ʒ���������Ƶ���������________��
���𰸡�H2SO4+2NaOH�TNa2SO4+2H2O���Ȼ�����Һ��2Al+2NaOH+2H2O�T2NaAlO2+3H2����e��������ɫ�����������ڢ٢ݢۢܣ�����Ʒ���������Ƶ���������47%��
��������
�⣺(1)��ϡ�������������Ʒ������кͷ�Ӧ�����������ƺ�ˮ���÷�Ӧ�Ļ�ѧ����ʽ��H2SO4+2NaOH�TNa2SO4+2H2O���ڸ�ʵ�����û��������������Ӧ�����Һ�еμӷ�̪��Һ����Һ����ɫ��˵������Һ��û���������ƣ���Һ�����Ի����ԡ�ͨ������ʯ����Һ������ͭ���塢̼������Һ���ܼ�����Ƿ������ᣬ����ϡ���ᡢ�����ƶ������Ȼ�����Һ��Ӧ�����˰�ɫ�����������Ȼ�����Һ���ܴﵽʵ��Ŀ�ģ�
(2)�������������ƺ�ˮ������Ӧ������ƫ�����ƣ�NaAlO2����һ����Է���������С�����嵥�ʣ�����������÷�Ӧ�Ļ�ѧ����ʽΪ��2Al+2NaOH+2H2O�T2NaAlO2+3H2����
(3)������ʵ���У�Na2CO3��KOHû�г������ɣ����ܷ������ֽⷴӦ����ʵ��c����������������ͭ��Ӧ������������ͭ��ɫ�������ɹ۲쵽��������������ɫ������
(4)������������һ�����ô����dz�����������̣���ʹ�������Ȼ���ƫ�٣��ᵼ����������Һ����������������7%�����������Dz����Ǽ��㡢��������ȡ���ܽ⣬������ȷ��˳��Ϊ���ڢ٢ݢۢ���
(5)���ɵĶ������۵�����Ϊ��10g+50g-57.8g=2.2g����̼���Ƶ�����Ϊx��
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 44
x 2.2g
![]()
x=5.3g
��Ʒ���������Ƶ����������ǣ�![]() ��100%=47%��
��100%=47%��