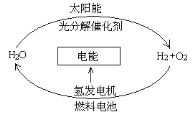
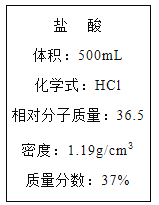
��Ŀ����
����Ŀ�������ͼ�ش���ȡ��������֤��������ʵ����й����⡣
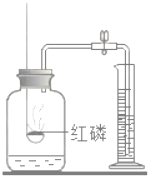
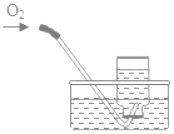
(1)д��Bͼ������������(������ľ�顢��)_______��_______��_______��
(2)���ȸ������������ʱ��ѡ�õķ���װ����_______���ռ�װ����_______��
(3)B�Թ��ڵĵ��ܿڴ�������һ��������Ŀ����_______��
(4)�������E�ռ����������������_______��
(5)ʵ������ȡ�������÷ֽ����������Һ�ķ������÷����������������ŵ㣬�磺_______ (�����)��
�ٲ�������Ⱦ���ڲ�����ȡ�������ȡ���������ֻ������
���𰸡��Թܾƾ�������̨BC��E��ֹ������ط�ĩ��O2�����뵼���ܽ������ǵ�ľ�����ڼ���ƿ�������ľ����ȼ��֤���Ѽ����٢�
��������
��1��Bͼ������������Ϊ�Թܡ��ƾ��ơ�����̨��
��2�����ȸ��������ȡ�����ķ�Ӧ���״̬�ǹ�̬����Ӧ�������Ǽ��ȣ���ѡ�̹̼�����װ��B���������ܶȱȿ������ܶȴ�������ˮ���ʿ���ѡ�����ſ�����װ��E����ˮ��װ��C�ռ���
��3��������ؼ��ȷֽ�����ʽϿ죬��������ط�ĩ���뵼�����ܶ���������Ҫ���Թܿڷ�һ������
��4���������ſ������ռ������������������ô����ǵ�ľ������ƿ�ڣ��縴ȼ˵��������
��5���ù���������ȡ������ʵ�������ɵIJ�����ˮ����������������Ⱦ����Ӧ������ȣ��ʢ٢���ȷ���ۢܲ���ȷ����ѡ�٢ڡ�

 ��ɢ˼ά�¿���ϵ�д�
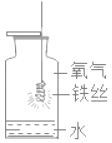
��ɢ˼ά�¿���ϵ�д�����Ŀ������ʵ��ָ�������е�ˮ�������û������ˮ����Ҫ���õ��ǣ� ��
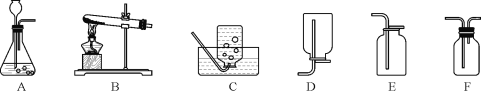
ʵ�� | A | B | C | D |
װ�� |
|
|
|
|
���� | ����ƿ�е�ˮ�����շų������� | ��Ͳ�е�ˮ��ͨ��ˮ������仯�ó�O2����� | ����ƿ�е�ˮ����ȴ������������ֹ����ƿը�� | ����ƿ�е�ˮ��ˮ�Ƚ�����ƿ�ڵĿ����ž������ڹ۲�O2��ʱ�ռ��� |
A. AB. BC. CD. D