��Ŀ����
����Ŀ����ѧ������ѧ�û�ѧ�Ļ��������ߵĻ�ѧ�����ж�����;��
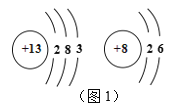
��1���û�ѧ���ű�ʾ��
��������ԭ��__________
������������______
�����������������_________
���������Ҫ�ɷ�_____
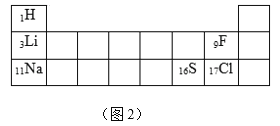
��2�������������ʣ�ѡ����Ӧ���ʵ���ĸ��գ�
A ��̿ B �ɱ� C ��ºϽ� D ��
��_____________��������ҵ������ԭ��
��___________���¶ȼ��г��õĽ���
��____________�������˹�����
��____________����������˿
��3����Ҫ��д���йػ�ѧ����ʽ
����˿��������ȼ��___________________��
��п��ϡ���ᷴӦ___________________��
��һ����̼��ԭ����������____________��
����������ȷֽ�����N2O�����ˮ______��
���𰸡�2Al 3N2 H2O Fe2O3 A D B C 3Fe+2O2![]() Fe3O4 Zn+ H2SO4 =ZnSO4 + H2�� Fe3O4+4CO
Fe3O4 Zn+ H2SO4 =ZnSO4 + H2�� Fe3O4+4CO![]() 3Fe+4CO2 NH4NO3
3Fe+4CO2 NH4NO3![]() N2O+2H2O
N2O+2H2O
��������
��1����ԭ��ֱ����Ԫ�ط�������ʾ����Ҫ��ʾ���ԭ�ӣ���Ԫ�ط��ŵ�ǰ���������ʾԭ�ӵĸ�����2����ԭ�ӱ�ʾΪ2Al�����2Al��
�ڻ�ѧʽ����Ԫ�ط��ź����½ǵ������������ʾ����Ҫ��ʾ������ӣ��ڻ�ѧʽ��ǰ�������������ʾ��3�������ӱ�ʾΪ3N2�����3N2��
�������к�������������ˮ�����H2O��
���������Ҫ�ɷ��������������Fe2O3��
��2���ٽ�̿�ǹ�ҵ�Ͽ�������ұ�����������ʣ����A��
���¶ȼ��е�������ˮ�������D��
�۸ɱ��������˹����꣬���B��
����ºϽ��۵�ͣ������ڱ���˿�����C��
��3������˿��������ȼ���������������������3Fe+2O2![]() Fe3O4��
Fe3O4��
��п��ϡ���ᷴӦ��������п�����������Zn+ H2SO4 =ZnSO4 + H2����
��һ����̼��ԭ�����������������Ͷ�����̼�����Fe3O4+4CO![]() 3Fe+4CO2��
3Fe+4CO2��
����������ȷֽ�����N2O�����ˮ�����NH4NO3![]() N2O+2H2O��
N2O+2H2O��

 ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д� �����Ļ������������������ϵ�д�
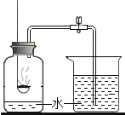
�����Ļ������������������ϵ�д�����Ŀ����˫�������dz��õ�ʳƷ���ʼ�������������������������̼��ˮ���������塣ij��ѧС���ͬѧ�ڴ�װʳƷ�з���һ�����õ�˫���������ǩ����ͼ��ʾ��

��������⣩���ù���ijɷ���ʲô��
���ռ����ϣ�
��1���������ϣ������Ȼ�����Һ�ڳ����������Ȼ�������Fe��2FeCl3��3FeCl2��
��2�������װ�۲죺���ַ�ĩ�ʺ�ɫ�����ַ�ĩ�ʰ�ɫ��������������ɫ�Ŀ�״���塣
���������룩���ù����п��ܺ���Fe��Fe2O3��CaO��Ca��OH��2��CaCO3����������п��ܺ���Fe2O3��������_____��
��ʵ��̽�����±��Ǽ���ͬѧ��Ʋ���¼��ʵ�鱨�棬���㲹��������
ʵ����� | ʵ������ | ʵ����� |
һ��ȡ�������������������ˮ�������ܽ� | ���岿���ܽ⣬���ų������� | ������һ������_____ |
�������ˣ�ȡ��Һ�μ���ɫ��̪��Һ | ��Һ���ɫ | ������һ�������������� |
����ȡ������������ϡ���� | ��������ʧ������������ɫ���壬�õ�dz��ɫ��Һ | ������һ������_____��һ��������Fe2O3 |
�ġ����������в���������ͨ�뵽����ʯ��ˮ�� | ����ʯ��ˮ����� | ������һ������_____ |
��ʵ�����ɣ�����ͬѧ��Ϊ����ͬѧ�ڶ��������ó��Ľ��۲�������������_____���û�ѧ����ʽ��ʾ��������ͬѧ�����������ó���һ��������Fe2O3���Ľ���Ҳ�Ǵ���ģ�������_____��
������̽����Ϊ��֤�������Ƿ���Fe2O3������ͬѧ�ô����ȷ�������ۣ�����������м�������ϡ���ᣬ����Һ��_____ɫ��֤�������к���Fe2O3��д���÷�Ӧ�Ļ�ѧ����ʽ_____��