��Ŀ����
Ϊ�ж�ij���ʴ��ڻ�ij����ǡ����ȫ��Ӧ��ͨ�����ض����ʵ���ɫ�ﵽĿ������ɫ�����ʾͳơ�ָʾ�������磺����ɫ��ʯ����ֽ����жϴ���Һ�����ԣ��õ�����Һ�����жϵ��ʵ⣨I2���Ĵ��ڣ�
��֪����SO2+I2+2H2O�TH2SO4+2HI����ɫ��Һ����
�ﳣ���£�������ˮ��
��5SO2+2H2O+KMnO4�TK2SO4+2H2SO4+2MnSO4����ɫ��Һ����
��1����ҵ�ϲ���SO2��N2��O2��������SO2��������ͼ����װ�ã�����װ��ʡ�ԣ���
�ٻ������ͨ��ͼʾ����װ��һ��ʱ�����Һ��ɫ����ɫ��Ϊ ɫʱ����ֹͣͨ�����˷����˲�SO2������ ��ѡ��͡��ߡ����Ļ�����壮
������װ���ڵ�I2�ĵ�����ҺһҲ������ ��Һ������Ϊָʾ������Ϊ ��
��2������10.0%������������Һ16.0g�μӵ�20.0g�����У���2�η�̪��Һ�������Һ�պ�����ɫ��Ϊ�ۺ�ɫʱ������Ϊǡ����ȫ��Ӧ��
��ԭ���������ʵ���������Ϊ ��
������ʽ����˵�����÷�Ӧ�����Һת��Ϊ20��ʱ������Һ��һ�ּ�������������ȷ��0.1g������֪��20��ʱ�Ȼ��Ƶ��ܽ��Ϊ36g��
1�����ޣ��ͣ��ڸ�����أ����������Һ����һ������SO2�����Ϻ�ɫ��Ϊ��ɫ����2����7.3%�ڿ�����Һ�м���9.8g�Ȼ���
����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�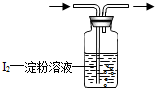 ��2012?���ݣ�Ϊ�ж�ij���ʴ��ڻ�ij����ǡ����ȫ��Ӧ��ͨ�����ض����ʵ���ɫ�ﵽĿ������ɫ�����ʾͳơ�ָʾ�������磺����ɫ��ʯ����ֽ����жϴ���Һ�����ԣ��õ�����Һ�����жϵ��ʵ⣨I2���Ĵ��ڣ�
��2012?���ݣ�Ϊ�ж�ij���ʴ��ڻ�ij����ǡ����ȫ��Ӧ��ͨ�����ض����ʵ���ɫ�ﵽĿ������ɫ�����ʾͳơ�ָʾ�������磺����ɫ��ʯ����ֽ����жϴ���Һ�����ԣ��õ�����Һ�����жϵ��ʵ⣨I2���Ĵ��ڣ� Ϊ�ж�ij���ʴ��ڻ�ij����ǡ����ȫ��Ӧ��ͨ�����ض����ʵ���ɫ�ﵽĿ������ɫ�����ʾͳơ�ָʾ�������磺����ɫ��ʯ����ֽ����жϴ���Һ�����ԣ��õ�����Һ�����жϵ��ʵ⣨I2���Ĵ��ڣ�
Ϊ�ж�ij���ʴ��ڻ�ij����ǡ����ȫ��Ӧ��ͨ�����ض����ʵ���ɫ�ﵽĿ������ɫ�����ʾͳơ�ָʾ�������磺����ɫ��ʯ����ֽ����жϴ���Һ�����ԣ��õ�����Һ�����жϵ��ʵ⣨I2���Ĵ��ڣ�
