��Ŀ����
Ϊ�ж�ij���ʴ��ڻ�ij����ǡ����ȫ��Ӧ��ͨ�����ض����ʵ���ɫ�ﵽĿ������ɫ�����ʾͳơ�ָʾ�������磺����ɫ��ʯ����ֽ����жϴ���Һ�����ԣ��õ�����Һ�����жϵ��ʵ⣨I2���Ĵ��ڣ�
��֪����SO2+I2+2H2O�TH2SO4+2HI����ɫ��Һ����
�ﳣ���£�������ˮ��
��5SO2+2H2O+KMnO4�TK2SO4+2H2SO4+2MnSO4����ɫ��Һ����
��1����ҵ�ϲ���SO2��N2��O2��������SO2��������ͼ����װ�ã�����װ��ʡ�ԣ���
�ٻ������ͨ��ͼʾ����װ��һ��ʱ�����Һ��ɫ����ɫ��Ϊ ɫʱ����ֹͣͨ�����˷����˲�SO2������ ��ѡ��͡��ߡ����Ļ�����壮
������װ���ڵ�I2�ĵ�����ҺһҲ������ ��Һ������Ϊָʾ������Ϊ ��
��2������10.0%������������Һ16.0g�μӵ�20.0g�����У���2�η�̪��Һ�������Һ�պ�����ɫ��Ϊ�ۺ�ɫʱ������Ϊǡ����ȫ��Ӧ��
��ԭ���������ʵ���������Ϊ ��
������ʽ����˵�����÷�Ӧ�����Һת��Ϊ20��ʱ������Һ��һ�ּ�������������ȷ��0.1g������֪��20��ʱ�Ȼ��Ƶ��ܽ��Ϊ36g��

1�����ޣ��ͣ��ڸ�����أ����������Һ����һ������SO2�����Ϻ�ɫ��Ϊ��ɫ����2����7.3%�ڿ�����Һ�м���9.8g�Ȼ���
��������������һ��ʵ��̽���ۺ��⣬����Ĺؼ��dz������������ṩ������Ϣ�������صĻ���������ɣ������漰������ϸߵ����ϵ͡���1���ٵ�ĵ�����Һ����ɫ������SO2+I2+2H2O�TH2SO4+2HI����ɫ��Һ����֪����Һ����ɫ�����ɫʱ�÷�Ӧǡ����ɣ���ʱӦ��ֹͣͨ�����壬ͨ����������ʹ���������ȷ�����ڸ�װ�����ն������������С�����Դ˷����˲�SO2�����ϵ͵Ļ�����壮
����Ϊ�Ϻ�ɫ������ص���Һͨ���������ʱ�ᷢ��5SO2+2H2O+KMnO4�TK2SO4+2H2SO4+2MnSO4����ɫ��Һ���ķ�Ӧ����Һ�������Ϻ�ɫ�����ɫ������Ҳ�ɲ��ø��������Һ�����ĵ�����Һ��ɸ�ʵ�顣
��2������ɿ�֪10.0%������������Һ16.0g��20.0g����ǡ����ȫ��Ӧ����˿�ͨ����Ӧ�ķ���ʽ����������������������м��㣬Ҳ�ɼ�������ɵ��Ȼ���������
������������������x�����ɵ��Ȼ���������y��
Na0H+HCl�TNaCl+H2O
40 36.5 58.5
16g��10% x y
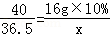
x=1.46g

�������������������Ϊ =7.3%��
=7.3%��
���ڸ���Һ��20��ʱ���������Կɲ��ü����ʵļ��취ʹ��ת���ɱ�����Һ��ԭ��Һ������20g+16g=36g��
��������ʵ�������z������ =
=
��z��9.8g

 ��������ϵ�д�
��������ϵ�д� ��ӡ�Ļ���ʱ����ϵ�д�
��ӡ�Ļ���ʱ����ϵ�д�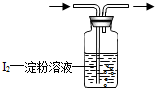 ��2012?���ݣ�Ϊ�ж�ij���ʴ��ڻ�ij����ǡ����ȫ��Ӧ��ͨ�����ض����ʵ���ɫ�ﵽĿ������ɫ�����ʾͳơ�ָʾ�������磺����ɫ��ʯ����ֽ����жϴ���Һ�����ԣ��õ�����Һ�����жϵ��ʵ⣨I2���Ĵ��ڣ�
��2012?���ݣ�Ϊ�ж�ij���ʴ��ڻ�ij����ǡ����ȫ��Ӧ��ͨ�����ض����ʵ���ɫ�ﵽĿ������ɫ�����ʾͳơ�ָʾ�������磺����ɫ��ʯ����ֽ����жϴ���Һ�����ԣ��õ�����Һ�����жϵ��ʵ⣨I2���Ĵ��ڣ� Ϊ�ж�ij���ʴ��ڻ�ij����ǡ����ȫ��Ӧ��ͨ�����ض����ʵ���ɫ�ﵽĿ������ɫ�����ʾͳơ�ָʾ�������磺����ɫ��ʯ����ֽ����жϴ���Һ�����ԣ��õ�����Һ�����жϵ��ʵ⣨I2���Ĵ��ڣ�
Ϊ�ж�ij���ʴ��ڻ�ij����ǡ����ȫ��Ӧ��ͨ�����ض����ʵ���ɫ�ﵽĿ������ɫ�����ʾͳơ�ָʾ�������磺����ɫ��ʯ����ֽ����жϴ���Һ�����ԣ��õ�����Һ�����жϵ��ʵ⣨I2���Ĵ��ڣ�
