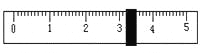
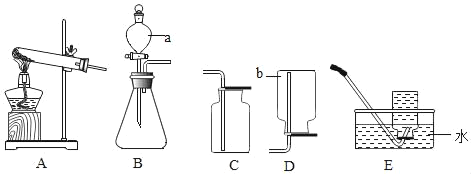
��Ŀ����
����Ŀ����ͭ��Zn����Ҫ����Ԫ�ص�ͭ�Ͻ𡣻�ͭ����Լ��Zn7%��ZnO31%��Cu50%��CuO5%������Ϊ���ʡ�������ͭ���ɵõ�����п������Ҫ�������£����ʲ�����ˮ�������뷴Ӧ����

��1����ҺA�еĽ�����������_____���ѧʽ����
��2��������а����IJ�����Ҫ�õ��IJ���������_____��
��3����ҺA������_____������ڡ�����С�ڡ����ڡ�����ҺC��������
��4��д��������еĻ�ѧ����ʽ_____��
���𰸡�Zn2+��Cu2+ ������ С�� Zn+CuSO4=Cu+ZnSO4
��������
��1���ɻ�ͭ���ijɷֿ�֪��п��ϡ���ᷴӦ��������п������������п�����ᷴӦ��������п��ˮ������ͭ�����ᷴӦ��������ͭ��ˮ�����еĽ�����������п���Ӻ�ͭ���ӣ���ѧʽ�ֱ��ǣ�Zn2+��Cu2+��
��2����ͼ��֪����������˳�ȥͭ�����ʣ�����������dz�ȥп���û�����ͭ�����������������ڹ��˺����������о��õ����������Dz�������
��3��������ͼ��֪��A��C�Ĺ����з����ķ�Ӧ�ǣ�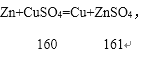
�ɴ˿ɼ���Һ�����������ˣ�������ҺA������С����ҺC��������
��4��������������֪��������еĻ�ѧ����ʽ�ǣ�Zn+CuSO4=Cu+ZnSO4��