��Ŀ����
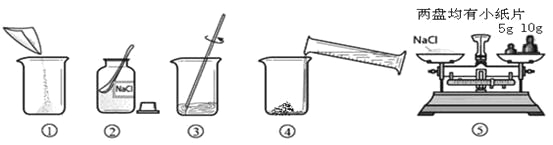
����Ŀ����ͼ������������������Ϊ10%��NaCl��Һ��ʵ�����ʾ��ͼ��
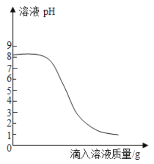
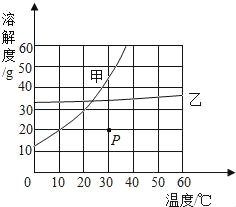
��1������ͼ��ʾ����ű�ʾ������Һ����ȷ����˳��_______________________��
��2��ͼ���У���һ��������������������___________��
��3������NaClʱ����ƽƽ����״̬��ͼ����ʾ��������ʾ������ͼ�����ȡ��NaCl����Ϊ______��
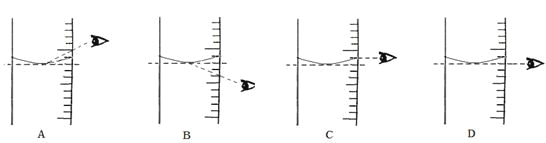
��4�����ݼ�����Ҫ��ȡˮ�������______��ˮ���ܶ�Ϊ1g/mL������ȡ����ʱ����ͼ���߽Ƕ���ȷ����_____����ѡ����ĸ��ţ�
��5������NaCl������ϷŻ�����ʱ��������һ������ȱ����һ��С�ǣ�����������������ȷ����������Һ��������������____������ڡ�����С�ڡ����ڡ���10%��
���𰸡��ڢݢ٢ܢ� ҩ�� 18.2g 90 mL D С��
��������
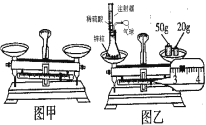
��1������������������һ������Һ�Ļ������裺���㡢��������ȡ���ܽ⡢װƿ��ţ�Ҫע���ڳ���ǰ��ȡ��ҩƷ���ܳ�������������Һ����ȷ����˳���ǣ��ڢݢ٢ܢ���
��2��ͼ���У���������Ϊȡ�÷�ĩ״ҩƷ��ҩ�ס�
��3������������ƽ���ݵĶ�����NaCl����Ϊ15g+3.2g=18.2g��
��4����������Һ�����ʵ�����=100g��10%=10g������Ҫˮ������=100g-10g=90g������Ҫ��ȡˮ������ǣ�90ml��ˮ���ܶ�Ϊ1g/mL����ʹ����Ͳ��ȡҺ��ʱ�������밼Һ����ʹ�������ͬһˮƽ���ϣ����߽Ƕ���ȷ���ǣ�D��
��5���������ȱ����һ��С�ǣ���������������ȷ����ôNaCl����С��С������ʵ����������ˮ��������ȷ�����������Һ��������������С��10%��

 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д� ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�