��Ŀ����
����A��F�dz��л�ѧ�е�����ʵ��װ�ã��밴Ҫ����գ�
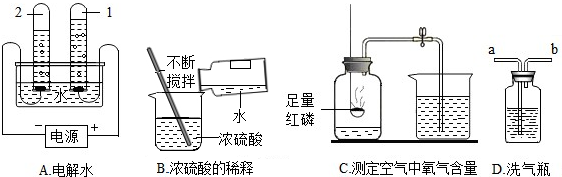
��1��Aʵ���Թ�2�в�����������
��2��Cʵ��˵�����������Լռ������
�������������
�ټ��װ�������� ��ʵ��ǰ�н�ֹˮ��
����ȴ���ٴ�ֹˮ�� ������۴������
��3������Dװ�ó�ȥO2�е�ˮ��������Һ���Լ�Ϊ
��4������ʵ���в��ܴﵽʵ��Ŀ����

��1��Aʵ���Թ�2�в�����������
����
����
���Թ�1��2��������������ԼΪ1��2
1��2
����2��Cʵ��˵�����������Լռ������
| 1 |
| 5 |
| 1 |
| 5 |
��
��
���ټ��װ�������� ��ʵ��ǰ�н�ֹˮ��
����ȴ���ٴ�ֹˮ�� ������۴������
��3������Dװ�ó�ȥO2�е�ˮ��������Һ���Լ�Ϊ
Ũ����
Ũ����
��ҽԺ���ô�װ�����۲�������������������b
b
���a����b���� Ӧ���Ӳ������������ܽ��ܣ���4������ʵ���в��ܴﵽʵ��Ŀ����
B
B
������ĸ������������1�����ݵ��ˮʵ�������ͽ��۷����ش�
��2�����ݲⶨ�������������������ʵ���ע������ͽ��۷����жϣ�
��3��Ũ���������ˮ�ԣ����������������װ��D�Ĺ��������÷�����
��4������ʵ���ԭ����ע���ʵ�ַ�����
��2�����ݲⶨ�������������������ʵ���ע������ͽ��۷����жϣ�
��3��Ũ���������ˮ�ԣ����������������װ��D�Ĺ��������÷�����
��4������ʵ���ԭ����ע���ʵ�ַ�����
����⣺��1��װ��A�ǵ��ˮ��ʵ�飬�Թ�1�ռ��ĵ�Դ�ĸ�������������϶࣬���������Թ�2�ռ��ĵ�Դ������������������٣����������Թ�1��2��������������ԼΪ�����1��2��
��2��װ��C�Dzⶨ���������������������ʵ�飬��ʵ���֪���������Լռ������
����ʵ��ʱ��Ӧ�ȼ��װ�������ԣ�ʵ��ǰ�н�ֹˮ�У���Ӧ����ȴ���ٴ�ֹˮ�У���������������Ӧ�����˶����������壮ƿ��ѹǿû�б仯�����ܲ�����������������������
��3������Ũ���������ˮ�Կ�������������ԣ�����Dװ�ó�ȥO2�е�ˮ��������Һ���Լ�ΪŨ���ҽԺ�ô�װ�����۲������������װ��D��ʢ��ˮʱ���������ӵ���aͨ�룬����b���Ӳ������������ܽ��ܣ�ͨ���۲�װ�������ݵĿ����жϸ����������������
��4����ϡ��Ũ����ʱ��Ӧ��Ũ���ᵹ��ˮ�У��в��ɽ�ˮ����Ũ�����У����ԣ�����ʵ���в��ܴﵽʵ��Ŀ����B��
�ʴ�Ϊ����1��������1��2����2��
���ܣ���3��Ũ���b����4��B��
��2��װ��C�Dzⶨ���������������������ʵ�飬��ʵ���֪���������Լռ������
| 1 |
| 5 |
��3������Ũ���������ˮ�Կ�������������ԣ�����Dװ�ó�ȥO2�е�ˮ��������Һ���Լ�ΪŨ���ҽԺ�ô�װ�����۲������������װ��D��ʢ��ˮʱ���������ӵ���aͨ�룬����b���Ӳ������������ܽ��ܣ�ͨ���۲�װ�������ݵĿ����жϸ����������������
��4����ϡ��Ũ����ʱ��Ӧ��Ũ���ᵹ��ˮ�У��в��ɽ�ˮ����Ũ�����У����ԣ�����ʵ���в��ܴﵽʵ��Ŀ����B��
�ʴ�Ϊ����1��������1��2����2��
| 1 |
| 5 |
�����������е�ʵ�鶼�ǻ���ʵ�飮������ˮ�ĵ�⡢Ũ�����ϡ�͡�ϴ���ķ����������ĺ����ⶨ�ȣ�֪ʶ��࣬��ʵ�������Ҫ�нϸߵ��ж�������

��ϰ��ϵ�д�
�����Ŀ





