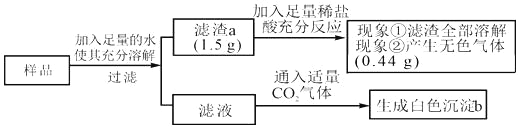
��Ŀ����
��һ����ɫ������Ʒ�����ܺ��� NaOH, Na2CO3, K2SO4, BaCl2 �е�һ�ֻ��֣� Ϊȷ������ɣ���������ʵ�飺
���� I��ȡ������Ʒ���ձ��У�������ˮ����ֽ��裬���ã��й��� A ʣ�ࡣȡ���� I �� ���ϲ���Һ�������Ϊ���ݣ��ֱ����ڲ��� II �Ͳ��� III �С�
���� II��ȡ���� I �����ϲ���Һ���Թ��У����뼸�� BaCl2 ��Һ��������ɫ������
���� III����ȡ���� I �����ϲ���Һ���Թ��У����뼸�η�̪��Һ����Һ���ɫ��
���� IV��ȡ���� I ʣ��Ĺ��� A������������ϡ���ᣬ���岿���ܽ⡣ ��������ʵ�飬�ش��������⣺
��1������ I �й��� A �ijɷ�Ϊ��_____��
��2����������ʵ���ƶϸû����ijɷ�,�϶����е���_____�����ܺ��е���_____��
��3����Ҫ��һ��ȷ����Ʒ����ɣ�������е�ʵ�������_____��
����ʵ���������ṩ�ķ������ܴﵽĿ�ĵ���
ѡ�� | ʵ��Ŀ�� | ʵ�鷽�� |
A | ϡ��Ũ���� | ��Ũ��������������ע��ˮ������Ͻ��� |
B | ������������ | �������ǵ�ľ�����뼯��ƿ�У����Ƿ�ȼ |
C | ��ij��Һ������ | �����̪��Һ���۲���Һ��ɫ�ı仯 |
D | ��ȥ�����Ȼ����������Ķ������� | ��ˮ�ܽ���ˣ����� |
A.A B.B C.C D.D
ʵ��������ƿû�б�ǩ����ɫ��Һ���ֱ���ϡ���ᡢ����������Һ������������Һ��̼������Һ�ͷ�̪��Һ��Ϊȷ����Һ��ɣ���ѧС�齫��ƿ��Һ���ΪA��B��C��D��E����������ʵ�顣
ʵ�� |
|
|
|
|
���� | ���������� | ��Һ��� | �������� | ��Һ��� |
(1)��̪��Һ�ı����_____��
(2)����ʵ�������������Һ������_____�����������Լ�����������д���������衢����ͽ��ۡ�_____��
(3)�ס�������С��ֱ��������ʵ���ʵ�������������Һ�����Լ�С��ķ�Һ�ס�
����۲쵽��Һ����ֻ����ɫ��Һ���������ʵ����Ϊ_____ (��д��̪����ͬ)��
����۲쵽��Һ������Һ�ʺ�ɫ�һ��ǣ����ϸ�����������ʵ����Ϊ__________ ��
�±��� 20��ʱ�������ʵ��ܽ�����ݡ�
���� | Ca��OH��2 | NaOH | CaCO3 | Ca��HCO3��2 | Na2CO3 | NaHCO3 |
�ܽ��/g | 0.16 | 109 | 0.0065 | 16.6 | 21.8 | 9.6 |
��1��������Һ������ 50g11%����������Һ�Ļ��������ǣ����㡪��ȡ�������ƹ��塪��ȡˮ���ܽ⡪װƿ����ǩ�� ��������ƽ��ȡ�������ƹ��������_________ g��
��2����������̽��ʵ�飬20��ʱ���������ݻش���������:
����ϡ����������Һ��ͨ�� CO2�������� Na2CO3������ͨ�� CO2��Na2CO3 ��ת��Ϊ NaHCO3,��֪����Ϊ���Ϸ�Ӧ����д���û��Ϸ�Ӧ�Ļ�ѧ����ʽ��_________�� ���� 20��ʱ���� Na2CO3 ��Һ��ͨ������� CO2���ɹ۲쵽��������_________��
�ڽ�ϱ����е��й����ݣ����� 20��ʱ��100g ������Һ���� CO2 ���������Ϊ���ݣ����ȥ CO �����е� CO2 ���ʣ�Ӧѡ��_________��Һ���ѧʽ����������CO2����Ĵ��ڣ���Ӧѡ��_________��Һ���ѧʽ����
�۽� 2.2g CO2 ͨ�� 47.8g һ��Ũ�ȵ� NaOH��Һ�г�ַ�Ӧ��Ӧ������û��CO2 �ݳ���ˮҲû������������Һ�е����ʽ��� Na2CO3�� NaHCO3 �������ʣ�NaHCO3 ��ˮ�е���� Na+�� HCO3-�������ʱ��Һ��̼Ԫ�ص���������Ϊ__________���ڴ˷�Ӧ�����У�����Ϊ�����ӵĸ���_________�����仯������д�� �л���û�У�
��3���絼�ʴ������ɸ���̽�����ֽⷴӦ��ʵ�ʡ���ͬ�����£�����Ũ��Խ�絼��Խ����Һ������Խǿ�������з�̪�� Ba(OH)2 ��Һƽ���ֳ��������������ձ��� ������絼�ʴ�������������һ�ݵμ�ϡ���ᣬ����һ�ݵμ���������Һ���μӹ����У���������Һ�ĵμ�����ʼ����ͬ�������Һ�ĵ絼�ʱ仯��ͼ��ʾ������˵����ȷ����__________
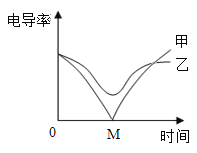
a�������ߵ絼�ʼ�С�����У���Һ�ɺ�ɫ��Ϊ��ɫ
b�������߶�Ӧ�ķ�Ӧ������������Ŀ������
c�������߶�Ӧ���������������Ʒ�Ӧ
d���������ϵ� M �����������Һǡ����ȫ��Ӧ
��ȥ��������������������ѡ�Լ��Ͳ�����������ȷ����
ѡ�� | ���� | ���� | �����Լ� | �������� |
A | Ag�� | Cu�� | AgNO3 | ���ˡ�ϴ�ӡ����� |
B | KCl���� | KClO3 | ������������ | ���� |
C | H2SO4��Һ | HCl��Һ | ����Ag NO3��Һ | ���� |
D | NH3 | ˮ���� | Ũ���� | ���� |
A.A B.B C.C D.D