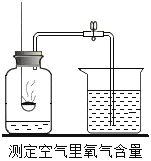
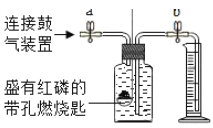
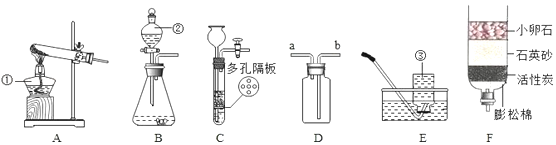
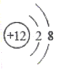
��Ŀ����
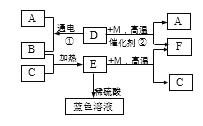
����Ŀ����ͼ��ʾijЩ���ʼ�ת����ϵ����֪D��һ�����Һ�壬CΪ�Ϻ�ɫ������EΪ��ɫ���塣A��B��M��FΪ���壬F�ܲ�����ɫֲ��Ĺ�����á���ش�
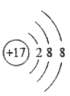
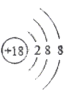
��1��E�Ļ�ѧʽ______��
��2����ɫ��Һ��һ�����е�������______________��д��ѧʽ����
��3��д�����з�Ӧ�Ļ�ѧ����ʽ����__________����__________��ʵ���ҽ��з�Ӧ��ʱ������������ϡ���ᣬ��Ŀ����__________��
��4����ҵ�Ͽ���A��F�ϳ��Ҵ���C2H6O������Ӧ��A��F��������Ϊ__________��
���𰸡� CuO CuSO4 2H2O ![]() 2H2��O2�� CO+H2O
2H2��O2�� CO+H2O ![]() CO2��H2 ��ǿ������ 3��22
CO2��H2 ��ǿ������ 3��22
�����������Һ����ˮ����D��H2O��CΪ�Ϻ�ɫ��������C��Cu��Cu�ڼ���ʱ����������Ӧ���ɺ�ɫ����������EΪ��ɫ���壬��E��CuO��B��O2��H2O��ͨ�������£������ֽⷴӦ������������������A��H2��FΪ���壬�ܲ�����ɫֲ��Ĺ�����ã���F��CO2����1��E�Ļ�ѧʽCuO����2�� CuO + H2SO4 ==CuSO4 + H2O��CuSO4������ˮ���γɵ���ҺΪ��ɫ������ɫ��Һ��һ�����е�������CuSO4����3����Ӧ�ٵĻ�ѧ����ʽ��2H2O![]() 2H2��+ O2������Ӧ�ڵĻ�ѧ����ʽΪ��CO+H2O
2H2��+ O2������Ӧ�ڵĻ�ѧ����ʽΪ��CO+H2O ![]() CO2��H2��������ˮ���������磬Ϊ����ǿˮ�ĵ����ԣ���ˮ�м�������ϡ���ᡣ��4����ҵ�Ϻϳ��Ҵ��Ļ�ѧ����ʽΪ��6H2 + 2CO2== C2H6O + 3H2O����Ӧ��H2��CO2��������Ϊ����6��2������2��44��=3:22��
CO2��H2��������ˮ���������磬Ϊ����ǿˮ�ĵ����ԣ���ˮ�м�������ϡ���ᡣ��4����ҵ�Ϻϳ��Ҵ��Ļ�ѧ����ʽΪ��6H2 + 2CO2== C2H6O + 3H2O����Ӧ��H2��CO2��������Ϊ����6��2������2��44��=3:22��