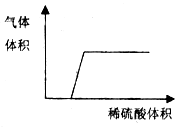
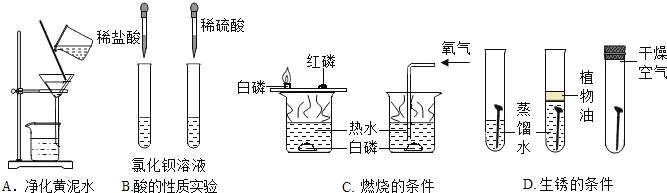
��Ŀ����
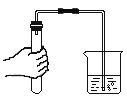
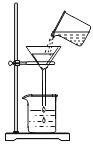
����Ŀ�������硱�Ǽ��ϳ��з�չ���ƶ��¾ɶ���ת������Ҫս�ԣ��������ڻƺ�Ⱥ�����¾ɶ���ת����������������ϰ桰�۰���������Ϊȫ���¾ɶ���ת����һ��·����Ҳ��־�ż��ϴӡ�������ʱ�������롰�ƺ�ʱ�������ƺ���������Ҫ�ij���ˮԴ��ij����С���ͬѧȡ�ƺ�ˮ�����������ͼ��ˮ������

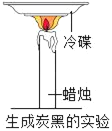
��1����ͼ3�������У�����̿��������_____��ѡ����ˡ���������������ɱ����֮һ��
��2����ͼ3�������У���������������_____��
��3����ͼ3�������У�������C12������ˮʱ��������ˮ��Ӧ��������ʹ����ᣨHClO������д���÷�Ӧ�Ļ�ѧ����ʽ_____��
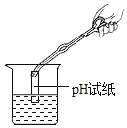
��4�����ճ������У�ͨ����ˮ���еμ�_____������Ӳˮ����ˮ��
��5��Ϊ�����ƺ�ˮԴ������˵������������ȷ����_____��
�����ƺ��ذ����۴�
�ڽ�ֹ�ذ�������ƺ����㵹��������
��ȡ���ƺ�����������ˮ����ҵ��ˮ���ۿڣ��½���ˮ������
��ȫ�������ȡ�ƺ��ذ��ľ�Ӫ����Ⱦ����
A�٢ڢ� B�ڢۢ� C�٢ۢ� D����ȫ��
���𰸡����� ���� Cl2+H2O��HCl+HClO ����ˮ B
��������
��1������̿���������ԣ�������ͼ3�������У�����̿������������ɫ�غ���ζ�����������
��2���ڹ��˲����У������������������������������
��3����������������ˮ��ɱ������������ˮ��Ӧ��������ʹ����ᣨHClO�������䷴Ӧ�Ļ�ѧ����ʽΪ��Cl2+H2O��HCl+HClO�����Cl2+H2O��HCl+HClO��
��4��Ӳˮ����ˮ���������������ĸ�þ���ӵĶ��٣������п��÷���ˮ������Ӳˮ����ˮ��������ĭ�϶������ˮ�����ٵ�Ӳˮ���������ˮ��
��5�������ƺ��ذ����۴���ˮ���������йأ�
�ڽ�ֹ�ذ�������ƺ����㵹����������ˮ���������йأ�
��ȡ���ƺ�����������ˮ����ҵ��ˮ���ۿڣ��½���ˮ��������ˮ���������йأ�
��ȫ�������ȡ�ƺ��ذ��ľ�Ӫ����Ⱦ������ˮ���������йأ�
���B��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�