��Ŀ����
����Ŀ��ˮ������������������Ҫ������:

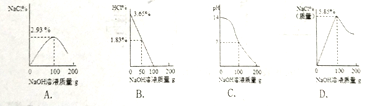
��1��ij��ͬѧͨ��ʵ���̽��ˮ����ɣ�ͨ��һ��ʱ����Թ�a���Թ�b������������ԼΪ________���Թ�b�е�������________��д��ѧʽ������Ӧ�Ļ�ѧ����ʽΪ_____________________��������ת���Ƕȷ��������ˮ��____��ת��Ϊ________�ܡ�
��2�����ˮʱҪ��ˮ�м�������ϡ������Һ����ǿˮ�ĵ����ԣ�����Ϊϡ������Һ�д��������ƶ���__________�������ӷ��ţ���
��3�����л���˵��ˮ������Ԫ�غ���Ԫ����ɵ�ʵ����________������ţ���
a������������ȼ�� bˮ���� cˮ�ľ���
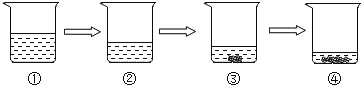
��4����һ��ͬѧ�����Ƽ���ˮ����ͼ����ʾ����������ˮ�����л���̿��������__________��������ij���ˮ���Ȫˮ������ˮ������ˮ����ˮ�����ڴ��������__________��
��5��С��ȡ���������������ˮ���Թ��У�������������ˮ���������н϶ม��������˵���������ˮ��_____ˮ������Ӳˮ��������ˮ�������ճ������г���________����ʹ��������ʹ�ã�
��6������ʵ���У�ˮ����������������_______������ĸ��ţ�
a  �ܽ����� b
�ܽ����� b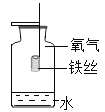 ��˿ȼ�� c
��˿ȼ�� c  �������
�������
���𰸡�2:1 O2 2H2O![]() 2H2
2H2![]() +O2
+O2![]() �� ��ѧ H+��SO42- a �������� ����ˮ Ӳ ��� c
�� ��ѧ H+��SO42- a �������� ����ˮ Ӳ ��� c
��������
(1)��ͼ��֪���Թ�a������������Թ�b�࣬�Թ�aΪ�������Թ�bΪ�����������������������Ϊ��2:1������д��2:1��
�Թ�bΪ����������д��O2��
ˮͨ����������������������д��2H2O![]() 2H2
2H2![]() +O2
+O2![]() ��
��
���ˮ��ͨ�磬��ˣ�������ת��Ϊ��ѧ�ܣ�����д���磻��ѧ��
(2)������ˮ������Ϊ�����ƶ��������Ӻ���������ӣ���ˣ����ˮʱҪ��ˮ�м�������ϡ������Һ����ǿˮ�ĵ����ԣ�����д��H+��SO42-��
(3) a��������������ȼ�գ�����ˮ������������Ԫ����ɣ�����������Ԫ����ɣ����������غ㶨�ɿ�֪��ˮ������Ԫ�غ���Ԫ����ɵģ��ʷ������⣻
b��ˮ�������������������仯������֤��ˮ������Ԫ�غ���Ԫ����ɣ��ʲ��������⣻
c��ˮ�ľ�������Ҫ�������������仯������֤��ˮ������Ԫ�غ���Ԫ����ɣ��ʲ��������⣻
(4)����̿�ṹ���ɶ�ף������������ã�����д���������ã�
����������һ��������ɣ�����ˮ���Ȫˮ������ˮ��������������������ɣ����ڻ�������ˮ����һ������ˮ��ɣ����ڴ��������д������ˮ��
(5)�������ˮ��������������ˮ���������н϶ม���������и���˵����ˮΪӲˮ������д��Ӳ��
�ճ������г�������еķ�����Ӳˮ����������д����У�
(6)a���ܽ�Ĺ����У�ˮ���ܼ� ���ʲ��������⣻
b����˿ȼ��ʵ���У�ˮ�������Ƿ�ֹ�������ヲ�伯��ƿ�ף�������ƿը�ѣ��ʲ��������⣻
c����������У�ˮ�������Ǹ����������ʷ������⡣

 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�����Ŀ����ѧ���о�����ת����ѧ�ʣ��DZ仯֮ѧ���������û�ѧ�仯���ϳɸ����²��ϣ���ϳ���ά���ϳ����ϣ��ϳ��ȣ�
��1����ͼ��ijƷ�Ʒ�װ��ǩ�IJ������ݣ�����ݱ�ǩ�ṩ����Ϣ�ش��������У������л��ϳ���ά����_____��

��2��������ë���Ϻͻ��˲��ϣ�
ʵ�鲽�� | ʵ������ | ʵ����� |
��ȡ������ƷһС�����ֱ����� | ��_____ | �ò�������ë���� |
��_____ | �ò����ǻ��˲��� |
��3�����ϳ���������ʳƷ����Ĥ��������ȣ�����ķ��������ǵ����������ʹ�ú����ⶪ�����������ɫ��Ⱦ����Ϊ�˱�����������������ɫ��Ⱦ���������ճ��������һ������������_____��
����Ŀ�������±��ش����⣮
�¶ȣ����� | 20 | 40 | 50 | 60 | 80 | |
�ܽ�ȣ�g/100gˮ�� | NaCl | 36.0 | 36.6 | 37.0 | 37.3 | 38.4 |
NH4Cl | 37.2 | 45.8 | 50.4 | 55.2 | 65.6 | |
KNO3 | 31.6 | 63.9 | 85.5 | 110 | 169 | |
��20��ʱ���ܽ������������_____��
��50��ʱ��100gˮ������ܽ�NaCl_____g��
��A��80������120gˮ��KNO3��Һ���������²������õ�102gKNO3���壮
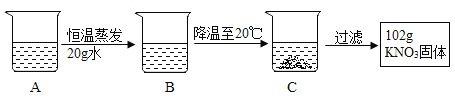
��1��A��ҺΪ_____��ѡ��������������������������Һ��
��2�������Ϲ��̵ķ�������ȷ����_____��ѡ���ţ���
A A��B�Ĺ����У���������û�иı�
B B���������ܼ���������Ϊ169��100
C ��ʼ����KNO3������¶���60����80��֮��
D A��Һ����������222g