��Ŀ����
��һ�������ĩ�����к���̼���ơ��������ơ�̼��ơ���ʯ�ҡ��Ȼ����е��������ʡ�ij��ȤС��Ϊȷ���������Ʋ���������ʵ�顣
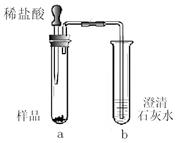
��ʵ��һ������ͬѧ��������̽��������ɱ��пհס�
��ʵ���������ͬѧ��������̽����

ʵ���ù���B�к�15 g��Ԫ�ء�
���ۺϼס�������ͬѧ��ʵ����з�������ա�(C-12 O-16 Na-23 Cl-35.5)
������ʵ����һ�������Ļ�ѧ��Ӧ�ǣ�Na2CO3+2HCl=2NaCl+H2O+CO2��
�Ƹù����ĩ����ɿ����� ��.
��ʵ��һ������ͬѧ��������̽��������ɱ��пհס�
| ʵ����� | ʵ������ | ʵ����ۼ����� |
| I��ȡ�����ù����ĩ���ձ��У�����������ˮ�ܽ⡢���� | ��ĩ�����ܽ⣬�õ���ɫ��������ɫ��Һ | ������һ������ |
| ������Һ�з�ĩ�����ܽ⣬�õ���ɫ������Һ | ��Һ��� | ��Һ�п��ܺ��� ����(����ԡ��������ԡ������ԡ�) |
| �������������Һ�μ�����ϡ���� | | ԭ�����ĩ��һ������Na2CO3 |

ʵ���ù���B�к�15 g��Ԫ�ء�
���ۺϼס�������ͬѧ��ʵ����з�������ա�(C-12 O-16 Na-23 Cl-35.5)
������ʵ����һ�������Ļ�ѧ��Ӧ�ǣ�Na2CO3+2HCl=2NaCl+H2O+CO2��
�Ƹù����ĩ����ɿ����� ��.
��̼��� ��д��ѧʽҲ�ɡ���ͬ��
����
�����������ݣ���Һ�ɺ�ɫ����ɫ
��1��CaCO3+2HCl = CaCl2+H2O+CO2��
��2��Na2CO3��NaCl��CaCO3��NaOH��Na2CO3��NaCl��CaCO3��CaO
����
�����������ݣ���Һ�ɺ�ɫ����ɫ
��1��CaCO3+2HCl = CaCl2+H2O+CO2��
��2��Na2CO3��NaCl��CaCO3��NaOH��Na2CO3��NaCl��CaCO3��CaO
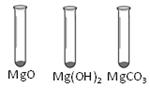
�����������ʵ��һ����ĩ�����ܽ⣬�õ���ɫ������˵������̼��ƣ���ĩ�����ܽ⣬�õ���ɫ��������Һ��ɺ�ɫ��˵����Һ�ʼ��ԣ����ܺ���̼���ƻ��������ƻ������ƣ�������̼���ƣ��������������ݣ��������ɵ����Ȼ��ƣ�����Һ�����ɺ�ɫ�����ɫ��
��ʵ���������ʵ��1��֪�������л�����̼��ƣ��ʼ�������ỹ����̼��Ʒ�Ӧ��CaCO3+2HCl = CaCl2+H2O+CO2������100 g15%��ϡ�����к�����Ԫ��100g��15%����35.5/36.5����100%=14.6g��С��ʵ���õ���Ԫ��15g���ʸ÷�ĩ��һ�������Ȼ��ƣ��ʸ�δ֪��ĩ����ɿ�����Na2CO3��NaCl��CaCO3��NaOH��Na2CO3��NaCl��CaCO3��CaO��

��ϰ��ϵ�д�
�����Ŀ