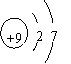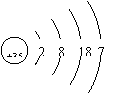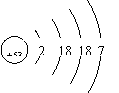��Ŀ����
| Ԫ������ | Ԫ�ط��� | ԭ�ӽṹʾ��ͼ | ���� ��ѧʽ |
���³�ѹ�µ��ʵ�״̬ | ���������� ��Ӧ��״�� |
| �� | F |  |
F2 | ��̬ ��̬ |
�ڰ�����ը |
| �� | Cl | 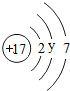 y= 8 8 |
Cl2 | ��̬ | ���ձ�ը |
| �� | Br | 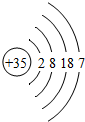 |
Br2 | Һ̬ | ���ȷ�Ӧ |
| �� | I |  x= 53 53 |
I2 I2 |
��̬ | �������Ȼ�����Ӧ |
��2�����Թ��ɷ����ȡ��塢���ԭ�ӽṹ���ɣ���һ�㼴�ɣ���
���Թ��ɷ����ȡ��塢���Ӧ���ʵ����ʱ仯���ɣ���һ�㼴�ɣ���
��2�������ϱ��ܽ���ɣ��ٹ��ɷ����ȡ��塢���ԭ�ӽṹ���ɣ�����������������ԭ�ӵĵ��Ӳ�����
�ڹ��ɷ����ȡ��塢���Ӧ���ʵ����ʱ仯���ɣ������������ķ�Ӧ���ҳ̶ȣ����ֵ��ʵĻ�ѧ������α仯��
��2�������ϱ��ܽ���ɣ����ɷ����ȡ��塢���ԭ�ӽṹ���ɣ��ٷ����ȡ��塢��ԭ�ӵ�������������ͬ������ȡ��塢��ԭ�ӵĵ��Ӳ���������
�ڹ��ɷ����ȡ��塢���Ӧ���ʵ����ʱ仯���ɣ������������ķ�Ӧ���ҳ̶ȣ��������ȡ��塢�ⵥ�ʵĻ�ѧ����������
�ʴ�Ϊ��
��1����̬��8 53 I2
��2�����������Ӷ���ͬ�����Ӳ������ϵ������ε�����
������ԭ�ӵĵ��Ӳ������ε�����ѧ����Խ��Խ�ȶ� �����������֣���

�Ƚ���������ɻ�ʹ���ͷ�Ա�ø�������������±�����Ϣ���ش��й����⣺
| Ԫ������ | Ԫ�ط��� | ԭ�ӽṹʾ��ͼ | ���� ��ѧʽ | ���³�ѹ�µ��ʵ�״̬ | ���������� ��Ӧ��״�� |
| �� | F |  | F2 | ______ | �ڰ�����ը |
| �� | Cl |  y=______ | Cl2 | ��̬ | ���ձ�ը |
| �� | Br |  | Br2 | Һ̬ | ���ȷ�Ӧ |
| �� | I |  x=______ | ______ | ��̬ | �������Ȼ�����Ӧ |
��2�����Թ��ɷ����ȡ��塢���ԭ�ӽṹ���ɣ���һ�㼴�ɣ���______��
���Թ��ɷ����ȡ��塢���Ӧ���ʵ����ʱ仯���ɣ���һ�㼴�ɣ���______��
�Ƚ���������ɻ�ʹ���ͷ�Ա�ø�������������±�����Ϣ���ش��й����⣺
| ���� | ���� | ԭ�ӽṹʾ��ͼ | ���ʻ�ѧʽ | ���³�ѹ��״̬ | ������������Ӧ |
| �� | F |
| F2 |
| �ڰ�����ը |
| �� | Cl |
| Cl2 | ��̬ | ���ձ�ը |
| �� | Br |
| Br2 | Һ̬ | ���ȷ�Ӧ |
| �� | I |
|
| ��̬ | �������Ȼ�����Ӧ |
��1������������еĿո�������
��2�����Թ��ɷ����ȡ��塢���ԭ�ӽṹ���ɣ���һ�㼴�ɣ���
��
���Թ��ɷ����ȡ��塢���Ӧ���ʵ����ʱ仯���ɣ���һ�㼴�ɣ���
��
��3����֪��Cl2��Br2�Ļ�ѧ�������ƣ�Cl2+H2O��HCl+HClO,����HClOҲ��һ���ᣬ��������������Һ��Ӧ�ķ���ʽΪ��HClO+NaOH��NaClO+ H2O���Էֱ�д��Br2��ˮ��Br2��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ�� ��
��