��Ŀ����
 ��2009?������һģ��2009��2�½���ʡij����������������ˮʱ�ŵ��̱ǵ�ũҩζ������⣬���г�������ˮ����ˮԴ�ܵ�ij�������ŷŵķ���������Ⱦ���������ڷ�����������һ���ж����ʣ��仯ѧʽΪ��C6H6O��������˵��������ǣ�������
��2009?������һģ��2009��2�½���ʡij����������������ˮʱ�ŵ��̱ǵ�ũҩζ������⣬���г�������ˮ����ˮԴ�ܵ�ij�������ŷŵķ���������Ⱦ���������ڷ�����������һ���ж����ʣ��仯ѧʽΪ��C6H6O��������˵��������ǣ�����������������̼Ԫ�صĻ����������л��һ����̼��������̼�ͺ�̼���������������������ݻ�ѧʽ��������и�Ԫ�ص������ȣ������ʵ���Է����������������и�Ԫ�ص�����������
����⣺
A�����Ӻ���̼Ԫ�أ������л����ȷ��
B��������̼��������Ԫ�ص�������Ϊ��12��6��1��6��16=36��3��8������6��6��1��6��6��1�DZ��ӷ�����ԭ�ӵĸ����ȣ��ʴ���
C��������̼Ԫ�ص���������Ϊ
��100%��76.6%����ȷ��
D�����ӵ���Է�������=12��6+1��6+16=94����ȷ��
��ѡB��
A�����Ӻ���̼Ԫ�أ������л����ȷ��
B��������̼��������Ԫ�ص�������Ϊ��12��6��1��6��16=36��3��8������6��6��1��6��6��1�DZ��ӷ�����ԭ�ӵĸ����ȣ��ʴ���
C��������̼Ԫ�ص���������Ϊ
| 12��6 |
| 12��6+1��6+16 |
D�����ӵ���Է�������=12��6+1��6+16=94����ȷ��
��ѡB��
������������Ҫ���黯ѧʽ��Ӧ�ã�Ҫϸ�ļ�����н��

��ϰ��ϵ�д�
�����Ŀ
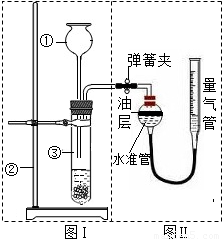 ��2009?������һģ��С���������ͼ��ʾ��ʵ��װ����֤������ͭ�ܼӿ����������Һ�ķֽ⣬������ͬ������MnO2�Ĵ�Ч�����Ƚϣ�ʵ��ʱ���������30 mL����Ϊ�����ȽϷ�Ӧ���ʣ�����ͼʾװ�ò�������������������������Ӱ��ʵ�����ؾ��Ѻ��ԣ�����������£�
��2009?������һģ��С���������ͼ��ʾ��ʵ��װ����֤������ͭ�ܼӿ����������Һ�ķֽ⣬������ͬ������MnO2�Ĵ�Ч�����Ƚϣ�ʵ��ʱ���������30 mL����Ϊ�����ȽϷ�Ӧ���ʣ�����ͼʾװ�ò�������������������������Ӱ��ʵ�����ؾ��Ѻ��ԣ�����������£�| ʵ����� | ����������Һ��� | ���� | �������� |
| �� | 15mL | �� | |
| �� | 15mL | CuO��0.5g�� | |
| �� | 15mL | MnO2��0.5g�� |
��2��ͼ����װ�����Ͳ��������______��
��3�������ԭ������ʵ���еġ��������ݡ�������ָ______��
��4��Ϊ�˽�ȷ�ز�������������ڶ�ȡ��Ӧǰ����������Һ��Ķ����Ĺ����У�Ӧע��______������ĸ��ţ���
a�������밼Һ����ʹ���ƽ
b������ǰ�����ƶ������ܺ�ˮ��
c������ˮ�ܾ�ֹ����ˮ����Һ�治������ʱ�����̶���
d������ǰӦ�����ƶ�ˮ�ܣ�������Һ����ƽ�ٶ���
��5�����Ҫ��һ��̽��CuO�Ƿ��Ǹ÷�Ӧ�Ĵ��������������ʵ�飮
| ʵ�鲽������� | �����һ�����Ŀ�� |
| ��1��ȡ15ml����������Һ������0.5gCuO����O2�����ʱ�δ����ʱ��Ķ� | CuO�ܼӿ����������Һ�ķֽ� |
| ______ | ______ |
| ______ | ______ |
��2009?������һģ��ͼ��a��b��c��d���ֹ������ʵ��ܽ�����ߣ��±�����Щ����������ijЩ�¶�ʱ���ܽ�ȣ�����ͼ����Ϣ���ж�����˵����ȷ���ǣ� ��
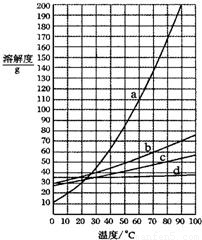
| NaCl | KCl | NH4Cl | KNO3 | |
| 10�� | 35.8g | 31.0g | 33.3g | 20.9g |
| 60�� | 37.3g | 45.5g | 55.2g | 110.0g |
