��Ŀ����
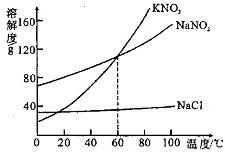 22����ͼΪKNO3��NaNO2���������ƣ���NaCl ���ܽ�����ߣ������ͼ��ش��������⣺
22����ͼΪKNO3��NaNO2���������ƣ���NaCl ���ܽ�����ߣ������ͼ��ش��������⣺��1��KNO3��NaNO2��NaCl�������ʵ��ܽ�����¶ȵ�Ӱ��������
KNO3
����2��KNO3��NaNO2���ܽ�����ʱ���¶���
60��
����3��NaNO2����ζ�������NaCl���ƣ���ʳNaNO2�������ж����������µķ����������ǣ�20��ʱ��ȡ�����ֹ����50g���ֱ���뵽100gˮ�У���ֽ��裬����û���ܽ������
NaCl
��������ȫ�ܽ����NaNO2
����4����Ҫʹ������3����û���ܽ���Ĺ�����ȫ�ܽ⣬Ӧ�ò�ȡ�ķ�����
��ˮ
����������1�����ܽ�������Ͽ��Կ������ʵ��ܽ�Ⱥ��¶ȵĹ�ϵ��
��2���������ʵ��ܽ�������ཻ�������ʾ�����ʵ��ܽ����ͬ��
��3��NaNO2��NaCl������ƣ������ܽ�����¶�Ӱ�첻ͬ�������ö����ܽ�Ȳ�ͬ������
��4��NaCl�ܽ�����¶�Ӱ��仯��С����Ҫʹδ�ܽ��NaCl�����ܽ⣬���Բ��������ܼ��ķ�����
��2���������ʵ��ܽ�������ཻ�������ʾ�����ʵ��ܽ����ͬ��
��3��NaNO2��NaCl������ƣ������ܽ�����¶�Ӱ�첻ͬ�������ö����ܽ�Ȳ�ͬ������
��4��NaCl�ܽ�����¶�Ӱ��仯��С����Ҫʹδ�ܽ��NaCl�����ܽ⣬���Բ��������ܼ��ķ�����
����⣺��1����ͼ�п���KNO3��б�����KNO3���ܽ�����¶�Ӱ�����
��2��60��ʱ��KNO3��NaNO2���ܽ�������ཻ���ʶ����ܽ�����ʱ���¶���60�棻
��3��60��ʱ��NaNO2���ܽ�ȴ���80g��NaCl�ܽ��С��40g��ȡ�����ֹ����50g���ֱ���뵽100gˮ�У���ֽ��裬NaNO2������ȫ�ܽ⣬NaCl����ȫ���ܽ⣻
��4��NaCl�ܽ�����¶�Ӱ��仯��С��Ҫʹδ�ܽ��NaCl�����ܽ⣬���Բ��ü�ˮ�ķ�����
�ʴ�Ϊ����1��KNO3����2��60�棻��3��NaCl��NaNO2����4����ˮ��
��2��60��ʱ��KNO3��NaNO2���ܽ�������ཻ���ʶ����ܽ�����ʱ���¶���60�棻
��3��60��ʱ��NaNO2���ܽ�ȴ���80g��NaCl�ܽ��С��40g��ȡ�����ֹ����50g���ֱ���뵽100gˮ�У���ֽ��裬NaNO2������ȫ�ܽ⣬NaCl����ȫ���ܽ⣻
��4��NaCl�ܽ�����¶�Ӱ��仯��С��Ҫʹδ�ܽ��NaCl�����ܽ⣬���Բ��ü�ˮ�ķ�����
�ʴ�Ϊ����1��KNO3����2��60�棻��3��NaCl��NaNO2����4����ˮ��
��������Ҫ�����˶Թ����ܽ�ȵĸ������������ܽ�����ߵ����壬�Դ�����ѧ������������������ѧ���������⡢��������������

��ϰ��ϵ�д�
�����Ŀ
 ��2007?��������ͼΪKNO3��NaNO2���������ƣ���NaCl���ܽ�����ߣ������ͼ��ش��������⣺
��2007?��������ͼΪKNO3��NaNO2���������ƣ���NaCl���ܽ�����ߣ������ͼ��ش��������⣺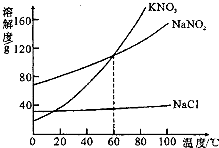 ��ͼΪKNO3��NaNO2���������ƣ���NaCl���ܽ�����ߣ������ͼ��ش��������⣺
��ͼΪKNO3��NaNO2���������ƣ���NaCl���ܽ�����ߣ������ͼ��ش��������⣺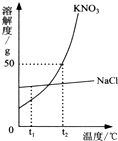 ��ͼΪKNO3��NaCl�������ʵ��ܽ�����ߣ�
��ͼΪKNO3��NaCl�������ʵ��ܽ�����ߣ�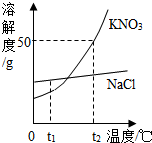 ��ͼΪKNO3��NaCl�������ʵ��ܽ�����ߣ�
��ͼΪKNO3��NaCl�������ʵ��ܽ�����ߣ�