��Ŀ����
��Ȼˮ�к��еIJ����������±���ʾ��
| �ܡ��⡡��� | �������� | ||
| ��Ҫ���� | ��Ҫ���� | ���������� | ϸ�������༰ԭ�� ���ɳ������� |
| ���� ������̼ ����� | �����ӡ������ӡ� ��������� þ���� | NH4+��NO2-�� HPO42-��Fe3+�� H2PO4-�� | |
��1��д������Ҫ���塱����������Ļ�ѧʽ________��________��
��2��д������Ҫ���ӡ���һ�������Ӻ�һ�������ӵķ���________��________��
��3��д���ɡ�����������е�������ɵ��������淋Ļ�ѧʽ________��
�⣺��1������Ϊ˫ԭ�ӷ��ӹ��ɵ����嵥�ʣ�����仯ѧʽΪO2��1��������̼������1��̼ԭ�Ӻ�2����ԭ�ӹ��ɣ�����仯ѧʽΪCO2��
��2�������Ӵ�2����λ������ɵ������ӣ������ӷ��ű�ʾΪCa2+�������Ӵ�1����λ�ĸ���ɵ������ӣ������ӷ��ű�ʾΪCl-��
��3��������������笠����Ӻ������������ӹ��ɵĻ�����������ӵĻ��ϼ۷ֱ�Ϊ+1��-1������仯ѧʽΪNH4H2PO4��
�ʴ�Ϊ����1��O2��CO2����2��Ca2+��Cl-����3��NH4H2PO4��
������˫ԭ�ӷ��ӹ��ɵ����嵥�ʵĻ�ѧʽ���ڶ�ӦԪ�ط������½DZ�ע����2��������Ļ�ѧʽ�ǰ������������˳��д����Ӧ��Ԫ�ط��ţ�����Ԫ�ط������½DZ�ע��Ӧ��ԭ�Ӹ��������ӷ�������Ԫ�ط��Ż�ԭ���ŷ��ŵ����ϽDZ�ע�����ĵ��������������ǰ�����������ں������ǡ�1��ʱʡ�ԣ�
������������Ҫ���黯ѧʽ����д�����ӷ��ŵ���д���Ѷ��Դ�
��2�������Ӵ�2����λ������ɵ������ӣ������ӷ��ű�ʾΪCa2+�������Ӵ�1����λ�ĸ���ɵ������ӣ������ӷ��ű�ʾΪCl-��
��3��������������笠����Ӻ������������ӹ��ɵĻ�����������ӵĻ��ϼ۷ֱ�Ϊ+1��-1������仯ѧʽΪNH4H2PO4��
�ʴ�Ϊ����1��O2��CO2����2��Ca2+��Cl-����3��NH4H2PO4��
������˫ԭ�ӷ��ӹ��ɵ����嵥�ʵĻ�ѧʽ���ڶ�ӦԪ�ط������½DZ�ע����2��������Ļ�ѧʽ�ǰ������������˳��д����Ӧ��Ԫ�ط��ţ�����Ԫ�ط������½DZ�ע��Ӧ��ԭ�Ӹ��������ӷ�������Ԫ�ط��Ż�ԭ���ŷ��ŵ����ϽDZ�ע�����ĵ��������������ǰ�����������ں������ǡ�1��ʱʡ�ԣ�
������������Ҫ���黯ѧʽ����д�����ӷ��ŵ���д���Ѷ��Դ�

��ϰ��ϵ�д�
�����Ŀ
2013��3��22���ǵڶ�ʮһ�조����ˮ�ա��� ����������ǡ�ˮ��������ˮ����������������������أ������°桶��������ˮ���������ӽ���7��1����ǿ��ʵʩ���±����ҹ��䲼����������ˮˮ�ʱ��IJ������ݣ�
��1���й�ָ����ֵ�������ˮ�� ���ʣ����������ѧ����������ѧָ���е� pH=8ʱ����������ˮ�� ������ԡ��������ԡ������ԡ�����
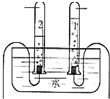
��ͼ��ʾij������ˮ�ľ������̣���ش��������⣺
��2����Ȼˮ�к����������ʣ����������������������˺�����ȷ������������о����̶���ߵķ����� ��
��3��Ӳˮ����������������ܶ��鷳�������п��� ������Ӳˮ����ˮ������ �ķ���������ˮ��Ӳ�ȣ�
��4����Һ������ɱ���Ĺ����У�ˮ�в�������������������ת��������ӣ������ӵķ����� ��
��5������ˮ����������Ŀǰ���õ�Һ�����⣬�������Ȱ���NH2Cl����������O3���ȣ�O3����Ԫ�صĻ��ϼ��� ��NH2Cl����Ԫ������Ԫ�ص��������� ��
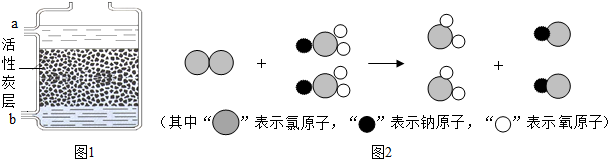
��6��ClO2����һ������ˮ����������������������Cl2��������ˮ����������ȡClO2�ķ�Ӧ����ʾ��ͼ���£�

�����У� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ�
�÷�Ӧ�Ļ�ѧ����ʽ�� ��

| ��Ŀ | �� |
| �й�ָ�� | ����ζ������� |
| ��ѧָ�� | pH 6.5��8.5��ͭ��1.0mg?L-1�������1.0mg?L-1�������ȡ�0.3mg?L-1�� |
��ͼ��ʾij������ˮ�ľ������̣���ش��������⣺
��2����Ȼˮ�к����������ʣ����������������������˺�����ȷ������������о����̶���ߵķ����� ��
��3��Ӳˮ����������������ܶ��鷳�������п��� ������Ӳˮ����ˮ������ �ķ���������ˮ��Ӳ�ȣ�
��4����Һ������ɱ���Ĺ����У�ˮ�в�������������������ת��������ӣ������ӵķ����� ��
��5������ˮ����������Ŀǰ���õ�Һ�����⣬�������Ȱ���NH2Cl����������O3���ȣ�O3����Ԫ�صĻ��ϼ��� ��NH2Cl����Ԫ������Ԫ�ص��������� ��
��6��ClO2����һ������ˮ����������������������Cl2��������ˮ����������ȡClO2�ķ�Ӧ����ʾ��ͼ���£�

������
 ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ��÷�Ӧ�Ļ�ѧ����ʽ�� ��

 ��2013?��ˮ��һģ��2013��3��22���ǵڶ�ʮһ�조����ˮ�ա��� ����������ǡ�ˮ��������ˮ����������������������أ������°桶��������ˮ���������ӽ���7��1����ǿ��ʵʩ���±����ҹ��䲼����������ˮˮ�ʱ��IJ������ݣ�
��2013?��ˮ��һģ��2013��3��22���ǵڶ�ʮһ�조����ˮ�ա��� ����������ǡ�ˮ��������ˮ����������������������أ������°桶��������ˮ���������ӽ���7��1����ǿ��ʵʩ���±����ҹ��䲼����������ˮˮ�ʱ��IJ������ݣ�
 ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ�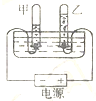
 ������ˮ�����������������Ȼ��Դ����������˽���٣�
������ˮ�����������������Ȼ��Դ����������˽���٣�