��Ŀ����
 ������ˮ�����������������Ȼ��Դ����������˽���٣�
������ˮ�����������������Ȼ��Դ����������˽���٣���1�������к��е��������������壬�����������ڴ������е�
��2������ʱ�̶��벻������������Ϊ�����е�������
��3����Ȼˮ�к��еIJ����������±���ʾ��
| �� �� �� �� | �������� | ||
| ��Ҫ���� | ��Ҫ���� | ���������� | ϸ�������༰ԭ�����ɳ������� |
| ���� ������̼ ����� |
�����ӡ������ӡ� ��������� þ���� |
NH4+��NO2-�� HPO42-��Fe3+�� H2PO4-�� | |
��д������Ҫ���塱����������Ļ�ѧʽ
��д������Ҫ���ӡ���һ�������ӵķ���
�ۡ�����������У�NH4+�������Ͻǵġ�+����ʾ��
����������ԭ�����ԴӺ�ˮ����ȡʳ�Σ�ʵ��������ʳ��ˮʱ�õ���������
�ٴ���Ȧ������̨ �ھƾ��� ��©�� �ܲ����� ����Ͳ ��������
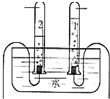
��4��С����ʵ�����������ˮʵ��ʱ��������ͼ��ʾ��������2���Թ����ӵĵ缫Ϊ
��������1�����ݵ��ʺͻ�����������ǣ���2���������������Ҫ���������ݹ�����õ�ԭ�ϣ������и��ɷ������������;�ش��⣻��3���ٸ��ݻ�ѧʽ����д������������ǰ�����ں�ʮ�ֽ�����Լ���������Ǵ�����ɵ����ӣ��������Ǵ�����ɵ����ӣ��۸������ӵ����壬���ϼ۵ļ��㷽�����ǣ��ܸ�������ʱ�õ������ƾ��ơ���Ҫ������̨֧�ţ������ò��������裻��4�����������������������������������������������������2��1����ͼʾ��֪2���Թ�����������������������������ӵĵ缫�Ǹ����������������˫ԭ�ӷ��ӣ�д�������Ļ�ѧʽ����������ȼ�յ�����ͷ���ʽ����д�����ش��⣮
����⣺��1�����ʺͻ�����������Ƿ���һ��Ԫ����ɣ�����������Ԫ��һ��Ԫ����ɣ����ڵ��ʣ�
��2�����������Ҫ��������������ʱ�̶��벻������������Ϊ�����е������ܹ���������ֲ����й������ԭ���Ƕ�����̼��ˮ��ѧʽ�ǣ�CO2��H2O��
�ڿ�����ռ78%��������N2���������ɵ����ӹ��ɵģ�����ͨ���⣬�����ڵ���У���������ԭ��ֱ�ӹ��ɣ����Ի�ѧʽ����Ԫ�ط���ֱ�ӱ�ʾ���ɱ�ʾΪ��He��ϡ������ԭ�ӵ�����������Ϊ8����Ϊ2�������ﵽ������ȶ��ṹ�����Ի�ѧ�����ȶ���
��3���ٻ�ѧʽ����д������������ǰ�����ں�ʮ�ֽ�����Լ����Ԫ����+1�ۣ���Ԫ����-2�ۣ����Ի�ѧʽ�ǣ�H2S��
���������Ǵ�����ɵ����ӣ��������Ǵ�����ɵ����ӣ��ɱ������ݿ�֪NH4+���������ӣ�NO2-���������ӣ�
��NH4+�������Ͻǵġ�+����ʾһ��笠����Ӵ�1����λ������ɣ�����Ԫ�ػ��ϼ���x��+1����2+x+��-2����4=-1���x=+5��
������ʱ�õ������ƾ��ơ���Ҫ������̨֧�ţ������ò��������裻
��4�������������������������������������������������2��1����ͼʾ��֪2���Թ�����������������������������ӵĵ缫�Ǹ����������������˫ԭ�ӷ��ӣ�����������ʾΪ��H2������ȼ�շ�������ɫ���棬���Ϸ���һ�ɶ�����ձ����ձ�������ˮ�����֣�˵����ˮ���ɣ���Ӧ������������������������ˮ���ù۲취��ƽ����Ӧ�����ǵ�ȼ�����Ա�ʾΪ��2H2+O2
2H2O��
�ʴ�Ϊ����1�����ʣ���2������������CO2��H2O�������ӣ�����ͨ���⣬���ڵ���У�He������������Ϊ8����Ϊ2���ﵽ������ȶ��ṹ����3����H2S����NH4+��NO2-����һ��笠����Ӵ�1����λ������ɣ�+5���ܣ��٢ڢܢޣ���4������H2������ɫ�����Ϸ���һ�ɶ�����ձ���
2H2+O2
2H2O��
��2�����������Ҫ��������������ʱ�̶��벻������������Ϊ�����е������ܹ���������ֲ����й������ԭ���Ƕ�����̼��ˮ��ѧʽ�ǣ�CO2��H2O��
�ڿ�����ռ78%��������N2���������ɵ����ӹ��ɵģ�����ͨ���⣬�����ڵ���У���������ԭ��ֱ�ӹ��ɣ����Ի�ѧʽ����Ԫ�ط���ֱ�ӱ�ʾ���ɱ�ʾΪ��He��ϡ������ԭ�ӵ�����������Ϊ8����Ϊ2�������ﵽ������ȶ��ṹ�����Ի�ѧ�����ȶ���
��3���ٻ�ѧʽ����д������������ǰ�����ں�ʮ�ֽ�����Լ����Ԫ����+1�ۣ���Ԫ����-2�ۣ����Ի�ѧʽ�ǣ�H2S��
���������Ǵ�����ɵ����ӣ��������Ǵ�����ɵ����ӣ��ɱ������ݿ�֪NH4+���������ӣ�NO2-���������ӣ�
��NH4+�������Ͻǵġ�+����ʾһ��笠����Ӵ�1����λ������ɣ�����Ԫ�ػ��ϼ���x��+1����2+x+��-2����4=-1���x=+5��
������ʱ�õ������ƾ��ơ���Ҫ������̨֧�ţ������ò��������裻
��4�������������������������������������������������2��1����ͼʾ��֪2���Թ�����������������������������ӵĵ缫�Ǹ����������������˫ԭ�ӷ��ӣ�����������ʾΪ��H2������ȼ�շ�������ɫ���棬���Ϸ���һ�ɶ�����ձ����ձ�������ˮ�����֣�˵����ˮ���ɣ���Ӧ������������������������ˮ���ù۲취��ƽ����Ӧ�����ǵ�ȼ�����Ա�ʾΪ��2H2+O2
| ||
�ʴ�Ϊ����1�����ʣ���2������������CO2��H2O�������ӣ�����ͨ���⣬���ڵ���У�He������������Ϊ8����Ϊ2���ﵽ������ȶ��ṹ����3����H2S����NH4+��NO2-����һ��笠����Ӵ�1����λ������ɣ�+5���ܣ��٢ڢܢޣ���4������H2������ɫ�����Ϸ���һ�ɶ�����ձ���
2H2+O2
| ||
�����������ؼ���Ҫ֪�����ʺͻ����������֪���������ú�����õĹ��̣���Ϥ�����и��ɷֵ������������;��֪����ѧʽ����д���������ӵı�ʾ������֪�����ˮʱ�������⣬�����һ��֪������ʽ��д��ע�����

��ϰ��ϵ�д�
 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д� ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�
�����Ŀ

