��Ŀ����
2013��3��22���ǵڶ�ʮһ�조����ˮ�ա�������������ǡ�ˮ��������ˮ����������������������أ�������ҹ��䲼����������ˮˮ�ʱ��IJ������ݣ�
��1���й�ָ����ֵ�������ˮ�� ���ʣ����������ѧ������
��2����Լ��ˮ����ֹˮ��ȾӦ��Ϊ���ǵ��Ծ���Ϊ�������й������д������
A���������������õ���ˮϰ�ߣ������ܳ������ÿһ��ˮ
B����ҵ��ˮ���������������ŷ�
C��ˮ����Ⱦ��Σ�����彡��
D������ʹ��ũҩ�����ʣ��������ˮ����Ⱦ
��3����ͼ1�ǻ���̿��ˮ��ʾ��ͼ������ˮ�еIJ��������ʣ�����̿�� ���ã�����ˮ�еĿ��������ʣ�����̿�� ���ã�
��4������Ӳˮ����ˮ���õ������� ��������ͨ�� �������Խ���ˮ��Ӳ�ȣ�
��5����ȷ��ˮ������Ԫ�غ���Ԫ����ɵ�ʵ����
A��������������ȼ������ˮ B��ˮ������
C��ˮ�ĵ�� D��ˮ�ľ���
��6��ˮͨ��ֽ��������������Դ--��������Ӧ�Ļ�ѧ����ʽΪ ��
��7����Ȼˮ�к����������ʣ����������������������˺�����ȷ������������о����̶���ߵķ����� ��
��8��ClO2����һ������ˮ�����������ҹ��ɹ����Ƴ���ȡClO2���·������䷴Ӧ���۹���ʾ��ͼ��ͼ2�����ݷ�Ӧ���۹���д����Ӧ�Ļ�ѧ���� ��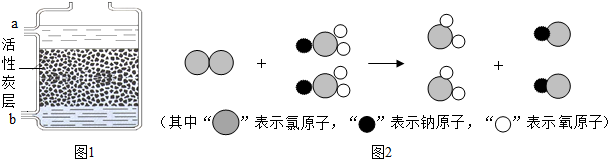
| ��Ŀ | �� |
| �й�ָ�� | ����ζ������� |
| ��ѧָ�� | pH��6.5��8.5��ͭ��1.0mg?L-1�������1.0mg?L-1�������ȡ�0.3mg?L-1�� |
��2����Լ��ˮ����ֹˮ��ȾӦ��Ϊ���ǵ��Ծ���Ϊ�������й������д������
A���������������õ���ˮϰ�ߣ������ܳ������ÿһ��ˮ
B����ҵ��ˮ���������������ŷ�
C��ˮ����Ⱦ��Σ�����彡��
D������ʹ��ũҩ�����ʣ��������ˮ����Ⱦ
��3����ͼ1�ǻ���̿��ˮ��ʾ��ͼ������ˮ�еIJ��������ʣ�����̿��
��4������Ӳˮ����ˮ���õ�������
��5����ȷ��ˮ������Ԫ�غ���Ԫ����ɵ�ʵ����
A��������������ȼ������ˮ B��ˮ������
C��ˮ�ĵ�� D��ˮ�ľ���
��6��ˮͨ��ֽ��������������Դ--��������Ӧ�Ļ�ѧ����ʽΪ
��7����Ȼˮ�к����������ʣ����������������������˺�����ȷ������������о����̶���ߵķ�����
��8��ClO2����һ������ˮ�����������ҹ��ɹ����Ƴ���ȡClO2���·������䷴Ӧ���۹���ʾ��ͼ��ͼ2�����ݷ�Ӧ���۹���д����Ӧ�Ļ�ѧ����

��������1�����ݸй�ָ����ֵ�����ˮ�����ʣ�����Ҫ������ѧ�仯�����ֽ��н��
��2�����ݽ�Լ��ˮ�ķ����Լ���ֹˮ��Ⱦ��ʩ���н��
��3�����ݻ���̿���������ԣ����Գ�ȥˮ��һЩ���������ʣ�ͬʱ����̿�㻹�����˲�����ã��˵�һЩ���������ʽ��н��
��4������Ӳˮ���н϶�ĸơ�þ���ӵĻ��������������������ܶ��鷳�������п��÷���ˮ������Ӳˮ����ˮ��Ӳˮ������ˮ��������ĭ�٣�����ˮ��������ĭ�࣬������еķ���������ˮ��Ӳ�Ƚ��н��
��5��������ȷ��ˮ������Ԫ�غ���Ԫ����ɵ�ʵ��Ҫ���������غ㶨�ɣ���Ӧǰ��Ԫ���������н��
��6������ˮͨ��ֽ������������������н��
��7����������ķ������Եõ���ˮ�����÷����Ǿ����̶���ߵĽ��н��
��8������������С���ʾ��ԭ�Ӽ��۹��̿�֪����Ӧ��Ϊ������NaClO2��������ΪClO2��NaCl���н��
��2�����ݽ�Լ��ˮ�ķ����Լ���ֹˮ��Ⱦ��ʩ���н��
��3�����ݻ���̿���������ԣ����Գ�ȥˮ��һЩ���������ʣ�ͬʱ����̿�㻹�����˲�����ã��˵�һЩ���������ʽ��н��
��4������Ӳˮ���н϶�ĸơ�þ���ӵĻ��������������������ܶ��鷳�������п��÷���ˮ������Ӳˮ����ˮ��Ӳˮ������ˮ��������ĭ�٣�����ˮ��������ĭ�࣬������еķ���������ˮ��Ӳ�Ƚ��н��
��5��������ȷ��ˮ������Ԫ�غ���Ԫ����ɵ�ʵ��Ҫ���������غ㶨�ɣ���Ӧǰ��Ԫ���������н��
��6������ˮͨ��ֽ������������������н��
��7����������ķ������Եõ���ˮ�����÷����Ǿ����̶���ߵĽ��н��
��8������������С���ʾ��ԭ�Ӽ��۹��̿�֪����Ӧ��Ϊ������NaClO2��������ΪClO2��NaCl���н��
����⣺��1���й�ָ����ֵ�����ˮ�����ʣ�����Ҫ������ѧ�仯�����֣����������ʣ����������
��2��A�������иı䲻������ˮϰ�ߣ������ܳ������ÿһ��ˮ�����������𣬽�Լ��ˮ����A��ȷ��
B����ҵ��ˮ���������������ŷŻ��ֹˮ��Ⱦ����B��ȷ��
C��ˮ����Ⱦ��Σ�����彡������C��ȷ��
D������ʹ��ũҩ�����ʣ��������ˮ����Ⱦ������Ҫ����ʹ�û��ʺ�ũҩ����D����ѡ��D��
��3������̿���������ԣ����Գ�ȥˮ��һЩ���������ʣ�ͬʱ����̿�㻹�����˲�����ã��˵�һЩ���������ʣ�������ˣ�������
��4��Ӳˮ���н϶�ĸơ�þ���ӵĻ��������������������ܶ��鷳�������п��÷���ˮ������Ӳˮ����ˮ��Ӳˮ������ˮ��������ĭ�٣�����ˮ��������ĭ�࣬������еķ���������ˮ��Ӳ�ȣ��������ˮ����У�
��5����ȷ��ˮ������Ԫ�غ���Ԫ����ɵ�ʵ��Ҫ���������غ㶨�ɣ���Ӧǰ��Ԫ������䣬������������ȼ������ˮ��ˮͨ����������������������˵��ˮ�к�����Ԫ�غ���Ԫ�أ����AC��
��6��ˮͨ��ֽ�������������������Ӧ�Ļ�ѧ����ʽΪ��2H2O
2H2��+O2�������2H2O
2H2��+O2����
��7������ķ������Եõ���ˮ�����÷����Ǿ����̶���ߵģ��������
��8��������С���ʾ��ԭ�Ӽ��۹��̿�֪����Ӧ��Ϊ������NaClO2��������ΪClO2��NaCl����Ӧ�ķ���ʽ�ǣ�Cl2+2NaClO2�T2ClO2+2NaCl�����Cl2+2NaClO2�T2ClO2+2NaCl��
��2��A�������иı䲻������ˮϰ�ߣ������ܳ������ÿһ��ˮ�����������𣬽�Լ��ˮ����A��ȷ��
B����ҵ��ˮ���������������ŷŻ��ֹˮ��Ⱦ����B��ȷ��
C��ˮ����Ⱦ��Σ�����彡������C��ȷ��
D������ʹ��ũҩ�����ʣ��������ˮ����Ⱦ������Ҫ����ʹ�û��ʺ�ũҩ����D����ѡ��D��
��3������̿���������ԣ����Գ�ȥˮ��һЩ���������ʣ�ͬʱ����̿�㻹�����˲�����ã��˵�һЩ���������ʣ�������ˣ�������
��4��Ӳˮ���н϶�ĸơ�þ���ӵĻ��������������������ܶ��鷳�������п��÷���ˮ������Ӳˮ����ˮ��Ӳˮ������ˮ��������ĭ�٣�����ˮ��������ĭ�࣬������еķ���������ˮ��Ӳ�ȣ��������ˮ����У�
��5����ȷ��ˮ������Ԫ�غ���Ԫ����ɵ�ʵ��Ҫ���������غ㶨�ɣ���Ӧǰ��Ԫ������䣬������������ȼ������ˮ��ˮͨ����������������������˵��ˮ�к�����Ԫ�غ���Ԫ�أ����AC��
��6��ˮͨ��ֽ�������������������Ӧ�Ļ�ѧ����ʽΪ��2H2O
| ||
| ||
��7������ķ������Եõ���ˮ�����÷����Ǿ����̶���ߵģ��������
��8��������С���ʾ��ԭ�Ӽ��۹��̿�֪����Ӧ��Ϊ������NaClO2��������ΪClO2��NaCl����Ӧ�ķ���ʽ�ǣ�Cl2+2NaClO2�T2ClO2+2NaCl�����Cl2+2NaClO2�T2ClO2+2NaCl��
�����������ȫ��ؿ�����ˮ�ľ�����֪ʶ���漰��֪ʶ��Ϲ㣬ֻ�н�ȫ��������йصĻ���֪ʶ�����������⣮

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ
 ��2013?��ˮ��һģ��2013��3��22���ǵڶ�ʮһ�조����ˮ�ա��� ����������ǡ�ˮ��������ˮ����������������������أ������°桶��������ˮ���������ӽ���7��1����ǿ��ʵʩ���±����ҹ��䲼����������ˮˮ�ʱ��IJ������ݣ�
��2013?��ˮ��һģ��2013��3��22���ǵڶ�ʮһ�조����ˮ�ա��� ����������ǡ�ˮ��������ˮ����������������������أ������°桶��������ˮ���������ӽ���7��1����ǿ��ʵʩ���±����ҹ��䲼����������ˮˮ�ʱ��IJ������ݣ�
 ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� 2013��3��22���ǵ�21�조����ˮ�ա����������������ǡ�ˮ��������Water Cooperation�������������ѧ���ش��������⣮
2013��3��22���ǵ�21�조����ˮ�ա����������������ǡ�ˮ��������Water Cooperation�������������ѧ���ش��������⣮