��Ŀ����
����Ҫ�����������գ�
��1����ľ�ҵ���Ҫ�ɷ���_____________���ѧʽ����ȡ���������̪��Һ����Һ��________ɫ��
��2����Ȼ������Ҫ�ɷ���____________�����������ƣ������ڿ�������ȫȼ�յĻ�ѧ����ʽΪ_______________________��
��3�����Ҫ��ȡ0.4mol������̼��������̼���___________mol����Ҫ��̼���80%�Ĵ���ʯ____________g��
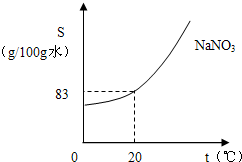
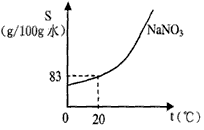
��4����ͼΪ�����Ƶ��ܽ�����ߡ�ʵ��������62.5g20%��������Һ�����к���������___________g����Ҫʹ����Һ��20��ʱ��ɱ�����Һ������Ҫ��____________g�����ơ������ƹ����У�����С�Ľ���������Һ����ú���ƻ����ϣ������_________ɫ��
��1����ľ�ҵ���Ҫ�ɷ���_____________���ѧʽ����ȡ���������̪��Һ����Һ��________ɫ��
��2����Ȼ������Ҫ�ɷ���____________�����������ƣ������ڿ�������ȫȼ�յĻ�ѧ����ʽΪ_______________________��
��3�����Ҫ��ȡ0.4mol������̼��������̼���___________mol����Ҫ��̼���80%�Ĵ���ʯ____________g��
��4����ͼΪ�����Ƶ��ܽ�����ߡ�ʵ��������62.5g20%��������Һ�����к���������___________g����Ҫʹ����Һ��20��ʱ��ɱ�����Һ������Ҫ��____________g�����ơ������ƹ����У�����С�Ľ���������Һ����ú���ƻ����ϣ������_________ɫ��
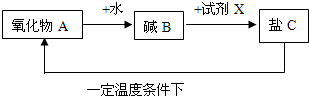
��5��ij������A�ܾ�������ѭ���仯��

���Լ�XΪ________���ѧʽ�����û�ѧ����ʽ��ʾ�ӡ���C��������Ӧ���ɡ�������A����_______________________��
��1��K2CO3 ����
��2�����飻CH4+2O2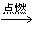 CO2+2H2O
CO2+2H2O
��3��0.4 ��50
��4��12.5 ��29 ����
��5��CO2��Na2CO3��K2CO3 ��CaCO3 CaO+CO2��
CaO+CO2��
��2�����飻CH4+2O2
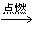 CO2+2H2O
CO2+2H2O ��3��0.4 ��50
��4��12.5 ��29 ����
��5��CO2��Na2CO3��K2CO3 ��CaCO3
 CaO+CO2��
CaO+CO2�� 
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ