��Ŀ����
����Ҫ�����������գ���1����ľ�ҵ���Ҫ�ɷ���
��2����Ȼ������Ҫ�ɷ���
��3�����Ҫ��ȡ0.4mol������̼��������̼���
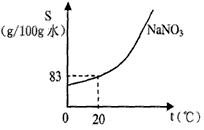
��4����ͼΪ�����Ƶ��ܽ�����ߣ�ʵ��������62.5g20%��������Һ�����к���������

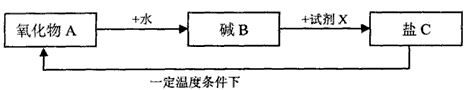
��5��ij������A�ܾ�������ѭ���仯��

���Լ�XΪ
�û�ѧ����ʽ��ʾ�ӡ���C��������Ӧ���ɡ�������A����
��������1����ľ�ҵ���Ҫ�ɷ�Ϊ̼��أ�����ˮ��Һ�ʼ��ԣ�����ȡ���������̪��Һ����Һ�ʺ�ɫ��
��2����Ȼ������Ҫ�ɷ��Ǽ��飬ȼ�����ɶ�����̼��ˮ��
��3�����ݷ�Ӧ�Ļ�ѧ����ʽ��CaCO3+2HCl=CaCl2+H2O+CO2������֪���Ҫ��ȡ1mol������̼��������̼���1mol�����ڣ���ȡ0.4mol������̼��������̼���0.4mol���Ӷ����̼��Ƶ�������
��4������֪62.5g20%��������Һ���������к��������Ƶ���������֪��20��ʱ���ܽ��Ϊ83�ˣ�������Ҫ����������ƹ��壻����������Һ�к��������ӣ���Ԫ��ȼ�շ��ƹ⣮
��5������Ŀ�Ʋ�������AΪ�����ƣ�BΪ�������ƣ�CΪ̼��ƣ��Լ�XֻҪ����̼�����������̼�����������ˣ�
��2����Ȼ������Ҫ�ɷ��Ǽ��飬ȼ�����ɶ�����̼��ˮ��
��3�����ݷ�Ӧ�Ļ�ѧ����ʽ��CaCO3+2HCl=CaCl2+H2O+CO2������֪���Ҫ��ȡ1mol������̼��������̼���1mol�����ڣ���ȡ0.4mol������̼��������̼���0.4mol���Ӷ����̼��Ƶ�������
��4������֪62.5g20%��������Һ���������к��������Ƶ���������֪��20��ʱ���ܽ��Ϊ83�ˣ�������Ҫ����������ƹ��壻����������Һ�к��������ӣ���Ԫ��ȼ�շ��ƹ⣮
��5������Ŀ�Ʋ�������AΪ�����ƣ�BΪ�������ƣ�CΪ̼��ƣ��Լ�XֻҪ����̼�����������̼�����������ˣ�
����⣺��1��̼���ˮ��Һ�ʼ��ԣ�����ȡ���������̪��Һ����Һ�ʺ�ɫ��
��2����Ȼ������Ҫ�ɷ��Ǽ��飬ȼ�����ɶ�����̼��ˮ�����Կ�д����ȼ�յĻ�ѧ����ʽ��
��3����ȡ0.4mol������̼��������̼���0.4mol����̼��Ƶ�����Ϊ100��0.4=40�ˣ���Ҫ��̼���80%�Ĵ���ʯΪ40��80%=50�ˣ�
��4����62.5��20%=12.5g��������Ҫ���������Ƶ�����Ϊx����
=
��x=29 ����Ԫ��ȼ�շ��ƹ�
��5������Ŀ�Ʋ�������AΪ�����ƣ�BΪ�������ƣ�CΪ̼��ƣ�
�ʴ�Ϊ����1��K2CO3�� ��2������ CH4+2O2
CO2+2H2O ��3��0.4 50 ��4��12.5 29 ��
��5��CO2��Na2CO3��K2CO3CaCO3
CaO+CO2��
��2����Ȼ������Ҫ�ɷ��Ǽ��飬ȼ�����ɶ�����̼��ˮ�����Կ�д����ȼ�յĻ�ѧ����ʽ��
��3����ȡ0.4mol������̼��������̼���0.4mol����̼��Ƶ�����Ϊ100��0.4=40�ˣ���Ҫ��̼���80%�Ĵ���ʯΪ40��80%=50�ˣ�
��4����62.5��20%=12.5g��������Ҫ���������Ƶ�����Ϊx����
| 12.5+x |
| 62.5+x |
| 83 |
| 100+83 |
��5������Ŀ�Ʋ�������AΪ�����ƣ�BΪ�������ƣ�CΪ̼��ƣ�
�ʴ�Ϊ����1��K2CO3�� ��2������ CH4+2O2
| ||
��5��CO2��Na2CO3��K2CO3CaCO3
| ||
���������⿼�����й���Һ���������ļ��㣬��Ҫ�˽ⳣ�����ʵ��ƶϣ������˽⻯ʯȼ�ϼ����ۺ����ã�

��ϰ��ϵ�д�
�����Ŀ