��Ŀ����
ʵ����������ͼ��ʾ��������
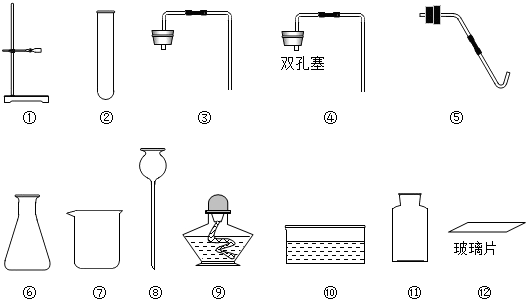
��1��д���������������ƣ�
�� ���� ��
��2��ʵ�����ü��ȸ�����صķ�����ȡ������ˮ���ռ���ƿO2���壬����ѡ���ˢ١��ڡ��ݡ��ᡢ�⣬��Ӧѡ�õ����������ǣ����ţ��� ��
������Ӧ�Ļ�ѧ����ʽ�ǣ� ��
��3��ʵ������ȡ������������Ҫ�������裺�ټ��Ȣڰ�ҩƷװ���Թܺ�̶�������̨�Ϣۼ��װ�õ������Ԣ�Ϩ��ƾ��Ƣ�����ˮ���ռ������ˮ����ȡ�����ܣ���ȷ�IJ���˳���ǣ�д��ţ� ��
��4��ijͬѧΪ��̽�����������Ի�ѧ��Ӧ���ʵ�Ӱ�죬������ͼ�е��������˶������̴���������ֽ��һ��ʵ�飺�й�ʵ���������±���
ͨ��������ʵ�����ݵķ�������ʵ����Եó��Ľ����ǣ� ��
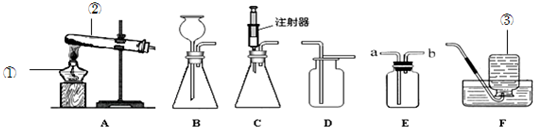
��5����6�֣��������ⲻ�ȶ�����Ȼ�ֽ⣬���õĹ���������Һ�������ʵ������������С��ij��ȤС��Ϊ�ⶨʵ������һƿ���õĹ���������Һ�����ʵ���������������ʵ�飮������������ͼ��ʾ��
�ٸ��������غ㶨�ɣ���Ӧ��������������Ϊ ��
�ڼ���ù���������Һ�����ʵ�������������д��������̣�

��1��д���������������ƣ�
��
��2��ʵ�����ü��ȸ�����صķ�����ȡ������ˮ���ռ���ƿO2���壬����ѡ���ˢ١��ڡ��ݡ��ᡢ�⣬��Ӧѡ�õ����������ǣ����ţ���
������Ӧ�Ļ�ѧ����ʽ�ǣ�
��3��ʵ������ȡ������������Ҫ�������裺�ټ��Ȣڰ�ҩƷװ���Թܺ�̶�������̨�Ϣۼ��װ�õ������Ԣ�Ϩ��ƾ��Ƣ�����ˮ���ռ������ˮ����ȡ�����ܣ���ȷ�IJ���˳���ǣ�д��ţ�
��4��ijͬѧΪ��̽�����������Ի�ѧ��Ӧ���ʵ�Ӱ�죬������ͼ�е��������˶������̴���������ֽ��һ��ʵ�飺�й�ʵ���������±���
| ʵ����� | ��1�� | ��2�� | ��3�� | ��4�� | ��5�� | ��6�� |
| MnO2��ĩ��������g�� | 0.1 | 0.3 | 0.5 | 0.7 | 0.9 | 1.1 |
| 10%��H2O2��Һ��������mL�� | 30 | 30 | 30 | 30 | 30 | 30 |
| �ռ������O2����ʱ�䣨S�� | 17 | 7 | 4 | 2 | 2 | 2 |
��5����6�֣��������ⲻ�ȶ�����Ȼ�ֽ⣬���õĹ���������Һ�������ʵ������������С��ij��ȤС��Ϊ�ⶨʵ������һƿ���õĹ���������Һ�����ʵ���������������ʵ�飮������������ͼ��ʾ��

�ٸ��������غ㶨�ɣ���Ӧ��������������Ϊ
�ڼ���ù���������Һ�����ʵ�������������д��������̣�
���㣺��������ȡװ��,�������ռ�����,��ȡ�����IJ��������ע���,�������ص��������,�й��������������ļ���,���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺�������������뻯ѧ����ʽ���ϵļ���,���������ʵ�����Ʒ������顢�����뾻��
��������1����Ϥ�������������ƺ���;��
��2��ʵ�����ü��ȸ�����صķ�����ȡ������ˮ���ռ���ƿO2���壬ѡ���������װ�ã����ݷ�Ӧд����Ӧ�Ļ�ѧ����ʽ��
��3��������ȡ�����Ĺ��̷����ش�
��4���۲�ͼ���������еı����Ƕ������̷�ĩ���ռ��������������ʱ�䣮Ȼ���ܽ���ۣ�
��5�����������غ㶨�ɻ������ٵ������������ɵ�������������
��6�����ݹ���������ȡ�����ķ���ʽ���������������������������������������������
��2��ʵ�����ü��ȸ�����صķ�����ȡ������ˮ���ռ���ƿO2���壬ѡ���������װ�ã����ݷ�Ӧд����Ӧ�Ļ�ѧ����ʽ��
��3��������ȡ�����Ĺ��̷����ش�
��4���۲�ͼ���������еı����Ƕ������̷�ĩ���ռ��������������ʱ�䣮Ȼ���ܽ���ۣ�
��5�����������غ㶨�ɻ������ٵ������������ɵ�������������
��6�����ݹ���������ȡ�����ķ���ʽ���������������������������������������������
����⣺��1���������֪��ͼ�������������ǣ�������ƿ�����dz���©����
��2�������������л���Ҫ�ռ����������Ӧѡ11��12������������ȷֽ���������غͶ������̺���������ƽ���ɣ�������Ӧ�Ļ�ѧ����ʽ�ǣ�2KMnO4
K2MnO4+MnO2+O2����
��3���������IJ����У����װ�õ������ԡ�װҩƷ�̶������ȡ��ռ������Ƴ����ܡ���Ϩ��ƾ��ƣ����Ƴ�������Ϩ��ƾ�����Ϊ�˷�ֹˮ������ը���Թܣ�����˳��Ϊ���ۢڢ٢ݢޢܣ�ˮ������ը���Թܣ�
��4���۲�ͼ����֪���������̷�ĩ����Խ���ռ��������������ʱ��Խ�̣�ͬʱ�������������̵������ӵ�һ��������Ӱ���������ֽ�����ʣ����Խ����ǣ����Ӷ������̵��������ܼӿ��������ֽ�����ʣ��������̵������ӵ�һ��������Ӱ���������ֽ�����ʣ�
��5���������غ㶨�ɻ������ٵ������������ɵ�������������Ϊ��33.0g+0.7g-33.9g=0.8g��
��6����������������Ϊx
2H2O2
2H2O+O2��
68 32
x 0.8g
=
��ã�x=1.7g
����������Һ�����ʵ���������Ϊ��
��100%=2%��
�ʴ�Ϊ����1����ƿ������©������2��11��12��2KMnO4
K2MnO4+MnO2+O2������3���ۢڢ٢ݢޢܣ�ˮ������ը���Թܣ���4�����Ӷ������̵��������ܼӿ��������ֽ�����ʣ��������̵������ӵ�һ��������Ӱ���������ֽ�����ʣ���5��0.8g����6��2%��
��2�������������л���Ҫ�ռ����������Ӧѡ11��12������������ȷֽ���������غͶ������̺���������ƽ���ɣ�������Ӧ�Ļ�ѧ����ʽ�ǣ�2KMnO4
| ||
��3���������IJ����У����װ�õ������ԡ�װҩƷ�̶������ȡ��ռ������Ƴ����ܡ���Ϩ��ƾ��ƣ����Ƴ�������Ϩ��ƾ�����Ϊ�˷�ֹˮ������ը���Թܣ�����˳��Ϊ���ۢڢ٢ݢޢܣ�ˮ������ը���Թܣ�
��4���۲�ͼ����֪���������̷�ĩ����Խ���ռ��������������ʱ��Խ�̣�ͬʱ�������������̵������ӵ�һ��������Ӱ���������ֽ�����ʣ����Խ����ǣ����Ӷ������̵��������ܼӿ��������ֽ�����ʣ��������̵������ӵ�һ��������Ӱ���������ֽ�����ʣ�
��5���������غ㶨�ɻ������ٵ������������ɵ�������������Ϊ��33.0g+0.7g-33.9g=0.8g��
��6����������������Ϊx
2H2O2
| ||
68 32
x 0.8g
| 68 |
| 32 |
| x |
| 0.8g |
����������Һ�����ʵ���������Ϊ��
| 1.7g |
| 34g |
�ʴ�Ϊ����1����ƿ������©������2��11��12��2KMnO4
| ||
��������������Ҫ����������Ʒ��Ͳ������裬ͬʱҲ���������������ƺͻ�ѧ����ʽ����д��������̼���������������������ȡ���ռ�װ�õ�ѡ���Լ���������������ͼ��鷽���ȶ����п�����Ҫ����֮һ�������ۺ��ԱȽ�ǿ����������Ҫ������ʵ�����У�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
�÷��ӵ�֪ʶ����������������������ȷ���ǣ�������
| A����1����ƶ���1����̶���ϵò���2�����϶���˵�����Ӽ���һ���ļ�� |
| B�����ǷŽ�ˮ�У���������ʧ����˵��������ˮ�������±�Ϊԭ�� |
| C�����ȵ���˿�ڿ������ڴ���������ͬ��˵�������е����������ʲ���ͬ |
| D��ˮ��ͨ������������������������˵�������ڻ�ѧ�仯�з����˱仯 |
���и����ʣ�������Ҫ�ɷ֣������ƻ��׳������Ļ�ѧʽ��һ�µ��ǣ�������
| A�����ʯ ʯī C |
| B���� �ɱ� H2O |
| C������ Һ�� O2 |
| D��ʯ��ʯ ����ʯ CaCO4 |
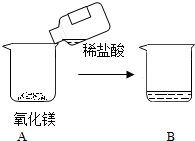 ��ѧ����ѧϰС��ͬѧʵ��̽������þ��ϡ���ᷴӦ����Һ�����ʳɷ֣�
��ѧ����ѧϰС��ͬѧʵ��̽������þ��ϡ���ᷴӦ����Һ�����ʳɷ֣�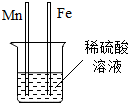 ����ͭ��������������������й㷺����;����ش��������⣺
����ͭ��������������������й㷺����;����ش��������⣺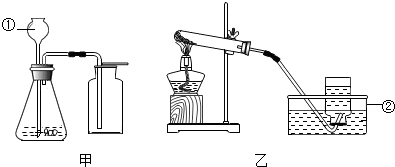
 Ϊ���ò������߲˵�ѧ��Ӫ�����⣬�ҳ���ե�õĸ�����ɫ���߲�֭�����������棬���ɿ�ζ���ء�Ӫ���ḻ���߲���ͷ��������ͷΪ�����ṩ��Ӫ������
Ϊ���ò������߲˵�ѧ��Ӫ�����⣬�ҳ���ե�õĸ�����ɫ���߲�֭�����������棬���ɿ�ζ���ء�Ӫ���ḻ���߲���ͷ��������ͷΪ�����ṩ��Ӫ������