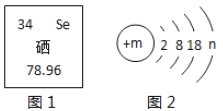
��Ŀ����
����Ŀ������ʦ�ڽ�����Һʱ���ù���M��������ʵ�飬ͼһ�мס��ҡ��������dz�ֽ�����õ�������ش��������![]() ����ˮ�Ļӷ�
����ˮ�Ļӷ�![]() ��
��
![]() ����ʵ������ж�����M���ܽ��������ͼ����Ӧ��______
����ʵ������ж�����M���ܽ��������ͼ����Ӧ��______![]() �����
�����![]() ��
��
![]() �ס��ҡ����ձ��е���Һһ���ʱ���״̬����______
�ס��ҡ����ձ��е���Һһ���ʱ���״̬����______![]() �����
�����![]() ��
��
![]() ����ͼ������Ҫʹ���ձ��е���Һǡ�ôﵽ����״̬������Ҫ����______g��M���塣
����ͼ������Ҫʹ���ձ��е���Һǡ�ôﵽ����״̬������Ҫ����______g��M���塣
![]() �ֱ�100gA��C�ı�����Һ��
�ֱ�100gA��C�ı�����Һ��![]() ���µ�
���µ�![]() ʱ����������Һ��������ȷ����______
ʱ����������Һ��������ȷ����______![]() �����
�����![]()
A A��C���DZ�����Һ B �����ܼ�������![]()
C ��Һ��������![]() D ���ʵ�����������
D ���ʵ�����������![]()
���𰸡�A �� 8 BD
��������
�⣺![]() ʱ�����ձ���50gˮ�ܽ����15g��û�й���ʣ�࣬����M���ʵ��ܽ����
ʱ�����ձ���50gˮ�ܽ����15g��û�й���ʣ�࣬����M���ʵ��ܽ����![]() ʱ�����ڻ����30g�������ж�����M���ܽ��������ͼ����Ӧ��A��
ʱ�����ڻ����30g�������ж�����M���ܽ��������ͼ����Ӧ��A��
![]() �ס������ձ��ײ�û�й���ʣ�࣬���Կ����DZ�����Һ�����ձ��ײ��й���ʣ�࣬����һ���DZ�����Һ��
�ס������ձ��ײ�û�й���ʣ�࣬���Կ����DZ�����Һ�����ձ��ײ��й���ʣ�࣬����һ���DZ�����Һ��
![]() ͨ�������ܽ�����߿�֪��
ͨ�������ܽ�����߿�֪��![]() ʱ��M���ʵ��ܽ����46g������Ҫʹ���ձ��е���Һǡ�ôﵽ����״̬������Ҫ����M����������ǣ�
ʱ��M���ʵ��ܽ����46g������Ҫʹ���ձ��е���Һǡ�ôﵽ����״̬������Ҫ����M����������ǣ�![]() ��
��
![]() �ֱ�100gA��C�ı�����Һ��
�ֱ�100gA��C�ı�����Һ��![]() ���µ�
���µ�![]() ʱ��
ʱ��
A.A�DZ�����Һ��C�Dz�������Һ����A����
B.�����¶ȣ�����Ӱ���ܼ������������������ܼ�������![]() ����B��ȷ��
����B��ȷ��
C.A���������壬C�����������壬������Һ��������![]() ����C����
����C����
D.A����![]() ʱ���ܽ�ȴ���C����
ʱ���ܽ�ȴ���C����![]() ʱ���ܽ�ȣ��������ʵ�����������
ʱ���ܽ�ȣ��������ʵ�����������![]() ����D��ȷ��
����D��ȷ��
��ѡ��BD��

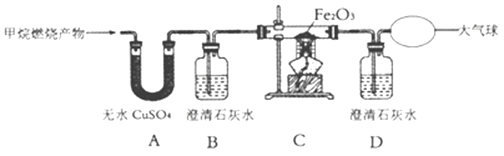
����Ŀ��ij��ѧ���ȤС������ͼװ�ý���һ����̼��ԭ����ͭ��̽��ʵ�飬��Ӧһ��ʱ��������ں�ɫ��ĩȫ����ɺ�ɫ������ʯ��ˮ����ǡ�
��1���Ӱ�ȫ�����ĽǶȿ��ǣ�����װ�õIJ���֮����_____��
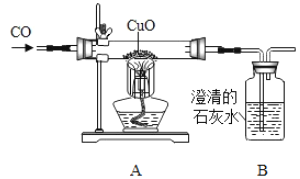
��2��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ_____��
��3��ͬѧ��ͨ���������ϵ�֪��������ͭ��Cu2O����ͭ��Ϊ��ɫ���壬��Cu2O�ܺ�ϡ���ᷴӦ����Ӧ�Ļ�ѧ����ʽΪCu2O+H2SO4��CuSO4+Cu+H2O�����ǶԷ�Ӧ�������еĺ�ɫ�����������̽����
��������⣩��Ӧ�����ɵĺ�ɫ����ɷ���ʲô��
���������룩����һ����ɫ������Cu�� ���������ɫ������Cu2O������������ɫ������_____��
��ʵ��̽�����������ʵ�鷽��
ʵ����� | ���� | ���� |
ȡ������ɫ�������Թ��У����������ϡ���ᡣ | ______ | ��ɫ���庬��Cu2O |
��˼�����������ͬѧ��Ϊ�������ȷ��ͬѧ�Ƿ�������Ϊ���Ľ��۲�ȷ��������_____��
Ϊ�ˣ�ͬѧ�Dz������������ʵ�飺��ȡmg��ɫ�������Թ��У����������ϡ�����ַ�Ӧ��Ȼ����ˡ�ϴ�ӡ�����������õ�ng���塣��n��_____����m�Ĵ���ʽ��ʱ�����Եó��������Ľ�����ȷ��
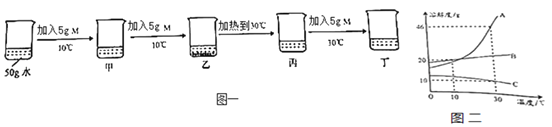
����Ŀ����ͼ�ǹ���������ܽ�����ߡ�
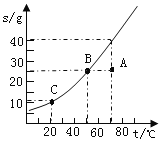
��1��ͼ��A�������_____��Һ��ѡ������������������������
��2������д�±�ʣ��Ŀո��ڱ��еĵ�1��2��3����ѡ���������������С�������������ڵ�������ѡ��������������������������
��Ŀ ���� | 1 | 2 | 3 | 4 | |
�����¶� | �ܼ� ���� | ���� ���� | ������������ | ��Һ״̬ | |
A��B | ���� | ���� | ���� | B�� | _____ |
B��C | ���� | _____ | _____ | C�� | _____ |